PPARγ and RhoBTB1 in hypertension
- PMID: 31789920
- PMCID: PMC7087486
- DOI: 10.1097/MNH.0000000000000579
PPARγ and RhoBTB1 in hypertension
Abstract
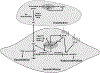
Purpose of review: This review provides an up-to-date understanding of how peroxisome proliferator activated receptor γ (PPARγ) exerts its cardioprotective effect in the vasculature through its activation of novel PPARγ target genes in endothelium and vascular smooth muscle.
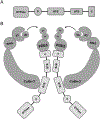
Recent findings: In vascular endothelial cells, PPARγ plays a protective role by increasing nitric oxide bioavailability and preventing oxidative stress. RBP7 is a PPARγ target gene enriched in vascular endothelial cells, which is likely to form a positive feedback loop with PPARγ. In vascular smooth muscle cells, PPARγ antagonizes the renin-angiotensin system, maintains vascular integrity, suppresses vasoconstriction, and promotes vasodilation through distinct pathways. Rho-related BTB domain containing protein 1 (RhoBTB1) is a novel PPARγ gene target in vascular smooth muscle cells that mediates the protective effect of PPARγ by serving as a substrate adaptor between the Cullin-3 RING ubiquitin ligase and phosphodiesterase 5, thus restraining its activity through ubiquitination and proteasomal degradation.
Summary: In the vasculature, PPARγ exerts its cardioprotective effect through its transcriptional activity in endothelium and vascular smooth muscle. From the understanding of PPARγ's transcription targets in those pathways, novel hypertension therapy target(s) will emerge.
Conflict of interest statement
Conflicts of interest
None
Figures
Similar articles
-
The 2023 Walter B. Cannon Award Lecture: Mechanisms Regulating Vascular Function and Blood Pressure by the PPARγ-RhoBTB1-CUL3 Pathway.Function (Oxf). 2024 Jan 5;5(1):zqad071. doi: 10.1093/function/zqad071. eCollection 2024. Function (Oxf). 2024. PMID: 38196837 Free PMC article. Review.
-
RhoBTB1 protects against hypertension and arterial stiffness by restraining phosphodiesterase 5 activity.J Clin Invest. 2019 Mar 21;129(6):2318-2332. doi: 10.1172/JCI123462. J Clin Invest. 2019. PMID: 30896450 Free PMC article.
-
Molecular mechanisms regulating vascular tone by peroxisome proliferator activated receptor gamma.Curr Opin Nephrol Hypertens. 2015 Mar;24(2):123-30. doi: 10.1097/MNH.0000000000000103. Curr Opin Nephrol Hypertens. 2015. PMID: 25587903 Free PMC article. Review.
-
Cullin-3 regulates vascular smooth muscle function and arterial blood pressure via PPARγ and RhoA/Rho-kinase.Cell Metab. 2012 Oct 3;16(4):462-72. doi: 10.1016/j.cmet.2012.08.011. Cell Metab. 2012. PMID: 23040068 Free PMC article.
-
Smooth Muscle Peroxisome Proliferator-Activated Receptor γ Plays a Critical Role in Formation and Rupture of Cerebral Aneurysms in Mice In Vivo.Hypertension. 2015 Jul;66(1):211-20. doi: 10.1161/HYPERTENSIONAHA.115.05332. Epub 2015 Apr 27. Hypertension. 2015. PMID: 25916724 Free PMC article.
Cited by
-
RBP7 functions as a tumor suppressor in HR + breast cancer by inhibiting the AKT/SREBP1 pathway and reducing fatty acid.Cancer Cell Int. 2024 Mar 29;24(1):118. doi: 10.1186/s12935-024-03299-0. Cancer Cell Int. 2024. PMID: 38553715 Free PMC article.
-
Methylation-Mediated Silencing of RBP7 Promotes Breast Cancer Progression through PPAR and PI3K/AKT Pathway.J Oncol. 2022 Oct 13;2022:9039110. doi: 10.1155/2022/9039110. eCollection 2022. J Oncol. 2022. PMID: 36276273 Free PMC article.
-
Kelch-like protein 3 in human disease and therapy.Mol Biol Rep. 2022 Oct;49(10):9813-9824. doi: 10.1007/s11033-022-07487-x. Epub 2022 May 18. Mol Biol Rep. 2022. PMID: 35585379 Review.
-
Machine learning analysis of FOSL2 and RHoBTB1 as central immunological regulators in knee osteoarthritis synovium.J Int Med Res. 2025 Apr;53(4):3000605251333646. doi: 10.1177/03000605251333646. Epub 2025 Apr 27. J Int Med Res. 2025. PMID: 40287984 Free PMC article.
-
Diversity of arterial cell and phenotypic heterogeneity induced by high-fat and high-cholesterol diet.Front Cell Dev Biol. 2023 Feb 23;11:971091. doi: 10.3389/fcell.2023.971091. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36910156 Free PMC article.
References
-
- Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10(4):355–61. - PubMed
-
- Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J Biol Chem. 1995;270(22):12953–6. - PubMed
-
- Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366(9493):1279–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

