Respiratory syncytial virus exhibits differential tropism for distinct human placental cell types with Hofbauer cells acting as a permissive reservoir for infection
- PMID: 31790466
- PMCID: PMC6886783
- DOI: 10.1371/journal.pone.0225767
Respiratory syncytial virus exhibits differential tropism for distinct human placental cell types with Hofbauer cells acting as a permissive reservoir for infection
Abstract
Background: Respiratory syncytial virus (RSV) is capable of transient viremia and extrapulmonary dissemination. Recently, this virus has been identified in fetal cord blood, suggesting the possibility of in utero acquisition in humans. Here, we assess permissivity and kinetics of RSV replication in primary human placental cells, examine their potential to transfer this infection to neighboring cells, and measure the inflammatory response evoked by the virus.
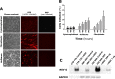
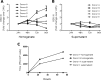
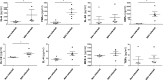
Methods and findings: Human placental villus tissue was collected immediately upon delivery and processed for isolation of placental cytotrophoblast, fibroblast, and macrophage (Hofbauer) cells. Isolated cells were infected with a recombinant RSV-A2 strain (rrRSV) expressing red fluorescent protein (RFP) and analyzed by fluorescence microscopy, Western blot, and quantitative PCR (qPCR). Based on RFP expression, rrRSV exhibited differential tropism for the three major placental cell types. Placental fibroblasts and Hofbauer cells were permissive and supported productive rrRSV replication. While infected cytotrophoblast cells expressed viral glycoprotein (G protein), only limited RSV replication was detected. Importantly, qPCR and fluorescence-focused unit assay revealed that the viral progeny remains trapped within infected Hofbauer cells for up to 30 days, with no release into surrounding media. Yet, Hofbauer cells passed the infection onto overlaid naïve 16HBE cells, suggesting contact-dependent trans-infection. Lastly, a significant increase in proinflammatory cytokines, particularly IL-6, TNF-alpha, and IFN-gamma was measured in the supernatant of infected Hofbauer cells by multiplex cytokine assay and conventional ELISA.
Conclusions: This study demonstrates that RSV can replicate in human placenta, exhibits differential tropism for distinct placental cell types, can be stored and transferred to neighboring cells by Hofbauer cells, and elicits an inflammatory response. It also supports the hypothesis that this respiratory virus can be vertically transferred to the fetus and potentially affect its development and the outcome of pregnancies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Campbell AP, Chien JW, Kuypers J, Englund JA, Wald A, Guthrie KA, et al. Respiratory virus pneumonia after hematopoietic cell transplantation (HCT): associations between viral load in bronchoalveolar lavage samples, viral RNA detection in serum samples, and clinical outcomes of HCT. J Infect Dis. 2010;201:1404–13. 10.1086/651662 - DOI - PMC - PubMed
-
- Waghmare A, Campbell AP, Xie H, Seo S, Kuypers J, Leisenring W, et al. Respiratory syncytial virus lower respiratory disease in hematopoietic cell transplant recipients: viral RNA detection in blood, antiviral treatment, and clinical outcomes. Clin Infect Dis. 2013;57:1731–41. 10.1093/cid/cit639 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

