A Pseudomonas aeruginosa type VI secretion system regulated by CueR facilitates copper acquisition
- PMID: 31790504
- PMCID: PMC6907878
- DOI: 10.1371/journal.ppat.1008198
A Pseudomonas aeruginosa type VI secretion system regulated by CueR facilitates copper acquisition
Abstract
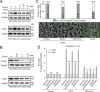
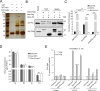
The type VI secretion system (T6SS) is widely distributed in Gram-negative bacteria, whose function is known to translocate substrates to eukaryotic and prokaryotic target cells to cause host damage or as a weapon for interbacterial competition. Pseudomonas aeruginosa encodes three distinct T6SS clusters (H1-, H2-, and H3-T6SS). The H1-T6SS-dependent substrates have been identified and well characterized; however, only limited information is available for the H2- and H3-T6SSs since relatively fewer substrates for them have yet been established. Here, we obtained P. aeruginosa H2-T6SS-dependent secretomes and further characterized the H2-T6SS-dependent copper (Cu2+)-binding effector azurin (Azu). Our data showed that both azu and H2-T6SS were repressed by CueR and were induced by low concentrations of Cu2+. We also identified the Azu-interacting partner OprC, a Cu2+-specific TonB-dependent outer membrane transporter. Similar to H2-T6SS genes and azu, expression of oprC was directly regulated by CueR and was induced by low Cu2+. In addition, the Azu-OprC-mediated Cu2+ transport system is critical for P. aeruginosa cells in bacterial competition and virulence. Our findings provide insights for understanding the diverse functions of T6SSs and the role of metal ions for P. aeruginosa in bacteria-bacteria competition.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
The T6SSs of Pseudomonas aeruginosa Strain PAO1 and Their Effectors: Beyond Bacterial-Cell Targeting.Front Cell Infect Microbiol. 2016 Jun 9;6:61. doi: 10.3389/fcimb.2016.00061. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27376031 Free PMC article. Review.
-
An Important Role of the Type VI Secretion System of Pseudomonas aeruginosa Regulated by Dnr in Response to Anaerobic Environments.Microbiol Spectr. 2022 Dec 21;10(6):e0153322. doi: 10.1128/spectrum.01533-22. Epub 2022 Oct 27. Microbiol Spectr. 2022. PMID: 36301114 Free PMC article.
-
Pseudomonas aeruginosa T6SS-mediated molybdate transport contributes to bacterial competition during anaerobiosis.Cell Rep. 2021 Apr 13;35(2):108957. doi: 10.1016/j.celrep.2021.108957. Cell Rep. 2021. PMID: 33852869
-
Initiation of H1-T6SS dueling between Pseudomonas aeruginosa.mBio. 2024 Aug 14;15(8):e0035524. doi: 10.1128/mbio.00355-24. Epub 2024 Jul 11. mBio. 2024. PMID: 38990002 Free PMC article.
-
Specialized killing across the domains of life by the type VI secretion systems of Pseudomonas aeruginosa.Biochem J. 2025 Jan 8;482(1):1-15. doi: 10.1042/BCJ20230240. Biochem J. 2025. PMID: 39774785 Free PMC article. Review.
Cited by
-
Machine learning from Pseudomonas aeruginosa transcriptomes identifies independently modulated sets of genes associated with known transcriptional regulators.Nucleic Acids Res. 2022 Apr 22;50(7):3658-3672. doi: 10.1093/nar/gkac187. Nucleic Acids Res. 2022. PMID: 35357493 Free PMC article.
-
Regulation of the H1 Type VI Secretion System by the Transcriptional Regulator NfxB in Pseudomonas aeruginosa.Int J Mol Sci. 2025 Feb 10;26(4):1472. doi: 10.3390/ijms26041472. Int J Mol Sci. 2025. PMID: 40003937 Free PMC article.
-
T6SS: A Key to Pseudomonas's Success in Biocontrol?Microorganisms. 2023 Nov 7;11(11):2718. doi: 10.3390/microorganisms11112718. Microorganisms. 2023. PMID: 38004732 Free PMC article. Review.
-
Transcriptional regulator Sar regulates the multiple secretion systems in Xanthomonas oryzae.Mol Plant Pathol. 2023 Jan;24(1):16-27. doi: 10.1111/mpp.13272. Epub 2022 Sep 30. Mol Plant Pathol. 2023. PMID: 36177860 Free PMC article.
-
Killing in the name of: T6SS structure and effector diversity.Microbiology (Reading). 2023 Jul;169(7):001367. doi: 10.1099/mic.0.001367. Microbiology (Reading). 2023. PMID: 37490402 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

