Fenfluramine for Treatment-Resistant Seizures in Patients With Dravet Syndrome Receiving Stiripentol-Inclusive Regimens: A Randomized Clinical Trial
- PMID: 31790543
- PMCID: PMC6902175
- DOI: 10.1001/jamaneurol.2019.4113
Fenfluramine for Treatment-Resistant Seizures in Patients With Dravet Syndrome Receiving Stiripentol-Inclusive Regimens: A Randomized Clinical Trial
Abstract
Importance: Fenfluramine treatment may reduce monthly convulsive seizure frequency in patients with Dravet syndrome who have poor seizure control with their current stiripentol-containing antiepileptic drug regimens.
Objective: To determine whether fenfluramine reduced monthly convulsive seizure frequency relative to placebo in patients with Dravet syndrome who were taking stiripentol-inclusive regimens.
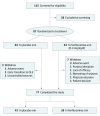
Design, setting, and participants: This double-blind, placebo-controlled, parallel-group randomized clinical trial was conducted in multiple centers. Eligible patients were children aged 2 to 18 years with a confirmed clinical diagnosis of Dravet syndrome who were receiving stable, stiripentol-inclusive antiepileptic drug regimens.
Interventions: Patients with 6 or more convulsive seizures during the 6-week baseline period were randomly assigned to receive fenfluramine, 0.4 mg/kg/d (maximum, 17 mg/d), or a placebo. After titration (3 weeks), patients' assigned dosages were maintained for 12 additional weeks. Caregivers recorded seizures via a daily electronic diary.
Main outcomes and measures: The primary efficacy end point was the change in mean monthly convulsive seizure frequency between fenfluramine and placebo during the combined titration and maintenance periods relative to baseline.
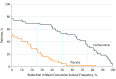
Results: A total of 115 eligible patients were identified; of these, 87 patients (mean [SD], age 9.1 [4.8] years; 50 male patients [57%]; mean baseline frequency of seizures, approximately 25 convulsive seizures per month) were enrolled and randomized to fenfluramine, 0.4 mg/kg/d (n = 43) or placebo (n = 44). Patients treated with fenfluramine achieved a 54.0% (95% CI, 35.6%-67.2%; P < .001) greater reduction in mean monthly convulsive seizure frequency than those receiving the placebo. With fenfluramine, 54% of patients demonstrated a clinically meaningful (≥50%) reduction in monthly convulsive seizure frequency vs 5% with placebo (P < .001). The median (range) longest seizure-free interval was 22 (3.0-105.0) days with fenfluramine and 13 (1.0-40.0) days with placebo (P = .004). The most common adverse events were decreased appetite (19 patients taking fenfluramine [44%] vs 5 taking placebo [11%]), fatigue (11 [26%] vs 2 [5%]), diarrhea (10 [23%] vs 3 [7%]), and pyrexia (11 [26%] vs 4 [9%]). Cardiac monitoring demonstrated no clinical or echocardiographic evidence of valvular heart disease or pulmonary arterial hypertension.
Conclusions and relevance: Fenfluramine demonstrated significant improvements in monthly convulsive seizure frequency in patients with Dravet syndrome whose conditions were insufficiently controlled with stiripentol-inclusive antiepileptic drug regimens. Fenfluramine was generally well tolerated. Fenfluramine may represent a new treatment option for Dravet syndrome.
Trial registration: ClinicalTrials.gov identifier: NCT02926898.
Conflict of interest statement
Figures
Comment in
-
You Can Teach an Old Drug New Tricks.Epilepsy Curr. 2020 May 12;20(4):193-195. doi: 10.1177/1535759720923035. eCollection 2020 Jul-Aug. Epilepsy Curr. 2020. PMID: 34025226 Free PMC article. No abstract available.
References
-
- Dravet C, Bureau M, Oguni H, Fukuyama Y, Cokar O. Severe myoclonic epilepsy in infancy: Dravet syndrome. Adv Neurol. 2005;95:71-102. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

