Benzothiazole derivatives as anticancer agents
- PMID: 31790602
- PMCID: PMC6896476
- DOI: 10.1080/14756366.2019.1698036
Benzothiazole derivatives as anticancer agents
Abstract
Benzothiazole (BTA) belongs to the heterocyclic class of bicyclic compounds. BTA derivatives possesses broad spectrum biological activities such as anticancer, antioxidant, anti-inflammatory, anti-tumour, antiviral, antibacterial, anti-proliferative, anti-diabetic, anti-convulsant, analgesic, anti-tubercular, antimalarial, anti-leishmanial, anti-histaminic and anti-fungal among others. The BTA scaffolds showed a crucial role in the inhibition of the metalloenzyme carbonic anhydrase (CA). In this review an extensive literature survey over the last decade discloses the role of BTA derivatives mainly as anticancer agents. Such compounds are effective against various types of cancer cell lines through a multitude of mechanisms, some of which are poorly studied or understood. The inhibition of tumour associated CAs by BTA derivatives is on the other hand better investigated and such compounds may serve as anticancer leads for the development of agents effective against hypoxic tumours.
Keywords: Benzothiazole; anticancer agent; carbonic anhydrase inhibitor; drug targets; scaffold.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures

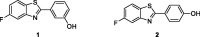



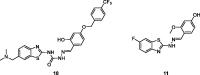
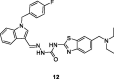
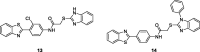





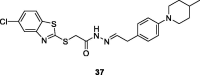
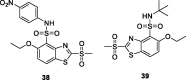
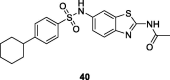
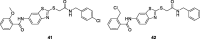



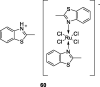
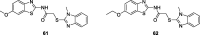


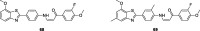
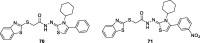
References
-
- Akhtar J, Khan AA, Ali Z.. Structure-activity relationship (SAR) study and design strategies of nitrogen-containing heterocyclic moieties for their anticancer activities. Eur J Med Chem 2017;5:143–89. - PubMed
-
- Keri SR, Patil RM, Patil AS, Budagumpi S.. A comprehensive review in current developments of benzothiazole-based molecules in medicinal chemistry. Eur J Med Chem 2015;89:207–51. - PubMed
-
- Gunawardana GP, Koehn FE, Lee AY, et al. . Pyridoacridine alkaloids from deep water marine sponges of the family Pachastrellidae: structure revision of dercitin and related compounds and correlation with the Kuanoniamines. J Org Chem 1992;57:1523–6.
-
- Jaiswal S, Mishra PA, Srivastava A.. The different kinds of reaction involved in synthesis of 2-substituted 2enzothiazole and its derivatives: a review. Res J Pharm Biol Chem Sci 2012;3:631–41.
-
- Bryson M, Fulton B, Benfield P.. Riluzole a review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in amyotrophic lateral sclerosis. Drugs 1996;52:549–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
