Teaching the basics of the mechanism of doxorubicin-induced cardiotoxicity: Have we been barking up the wrong tree?
- PMID: 31790851
- PMCID: PMC6909145
- DOI: 10.1016/j.redox.2019.101394
Teaching the basics of the mechanism of doxorubicin-induced cardiotoxicity: Have we been barking up the wrong tree?
Abstract
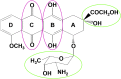
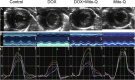
Doxorubicin (DOX), or Adriamycin, an anthracycline antibiotic discovered serendipitously as a chemotherapeutic drug several decades ago, is still one of the most effective drugs for treating various adult and pediatric cancers (breast cancer, Hodgkin's disease, lymphoblastic leukemia). However, one of the major side effects of the continuous use of DOX is dose-dependent, long-term, and potentially lethal cardiovascular toxicity (congestive heart failure and cardiomyopathy) in cancer survivors many years after cessation of chemotherapy. In addition, predisposition to cardiotoxicity varied considerably among individuals. The long-held notion that DOX cardiotoxicity is caused by reactive oxygen species formed from the redox-cycling of DOX semiquinone lacks rigorous proof in a chronic animal model, and administration of reactive oxygen species detoxifying agents failed to reverse DOX-induced cardiac problems. In this review, I discuss the pros and cons of the reactive oxygen species pathway as a primary or secondary mechanism of DOX cardiotoxicity, the role of topoisomerases, and the potential use of mitochondrial-biogenesis-enhancing compounds in reversing DOX-induced cardiomyopathy. New approaches for well-designed clinical trials that repurpose FDA-approved drugs and naturally occurring polyphenolic compounds prophylactically to prevent or mitigate cardiovascular complications in both pediatric and adult cancer survivors are needed. Essentially, the focus should be on enhancing mitochondrial biogenesis to prevent or mitigate DOX-induced cardiotoxicity.
Keywords: Cardio-oncology; Cardioprotection; Chemotherapy; Mitochondrial biogenesis; Reactive oxygen species; Topoisomerase.
Copyright © 2019. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Hardaway B.W. Adriamycin-associated cardiomyopathy: where are we now? updates in pathophysiology, dose recommendations, prognosis, and outcomes. Curr. Opin. Cardiol. 2019;34:289–295. - PubMed
-
- Young R.C., Ozols R.F., Myers C.E. The anthracycline antineoplastic drugs. N. Engl. J. Med. 1981;305:139–153. - PubMed
-
- Blum R.H., Carter S.K. Adriamycin. A new anticancer drug with significant clinical activity. Ann. Intern. Med. 1974;80:249–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

