Molecular Processes Implicated in Human Age-Related Nuclear Cataract
- PMID: 31791064
- PMCID: PMC7043214
- DOI: 10.1167/iovs.19-27535
Molecular Processes Implicated in Human Age-Related Nuclear Cataract
Abstract
Human age-related nuclear cataract is commonly characterized by four biochemical features that involve modifications to the structural proteins that constitute the bulk of the lens: coloration, oxidation, insolubility, and covalent cross-linking. Each of these is progressive and increases as the cataract worsens. Significant progress has been made in understanding the origin of the factors that underpin the loss of lens transparency. Of these four hallmarks of cataract, it is protein-protein cross-linking that has been the most intransigent, and it is only recently, with the advent of proteomic methodology, that mechanisms are being elucidated. A diverse range of cross-linking processes involving several amino acids have been uncovered. Although other hypotheses for the etiology of cataract have been advanced, it is likely that spontaneous decomposition of the structural proteins of the lens, which do not turn over, is responsible for the age-related changes to the properties of the lens and, ultimately, for cataract. Cataract may represent the first and best characterized of a number of human age-related diseases where spontaneous protein modification leads to ongoing deterioration and, ultimately, a loss of tissue function.
Figures
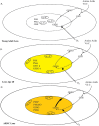
 . These attach to the inner surfaces of the fiber cell membranes, thus occluding the membrane pores. Proteins become colored, particularly in the lens center, as UV filters and other reactive metabolites (e.g., ascorbate) become bound and oxidize.
. These attach to the inner surfaces of the fiber cell membranes, thus occluding the membrane pores. Proteins become colored, particularly in the lens center, as UV filters and other reactive metabolites (e.g., ascorbate) become bound and oxidize.

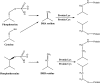
References
-
- Mukesh BN, Le A, Dimitrov PN, Ahmed S, Taylor HR, McCarty CA. Development of cataract and associated risk factors: the visual impairment project. Arch Ophthalmol. 2006;124:79–85. - PubMed
-
- Leske MC, Wu S-Y, Nemesure B, Hennis A. Risk factors for incident nuclear opacities. Ophthalmology. 2002;109:1303–1308. - PubMed
-
- Klein BE, Klein RE, Lee KE. Incident cataract after a five-year interval and lifestyle factors: the Beaver Dam eye study. Ophthalmic Epidemiol. 1999;6:247–255. - PubMed
-
- Truscott RJW. Age-related nuclear cataract—oxidation is the key. Exp Eye Res. 2005;80:709–725. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

