The impact of proline isomerization on antigen binding and the analytical profile of a trispecific anti-HIV antibody
- PMID: 31791173
- PMCID: PMC8675452
- DOI: 10.1080/19420862.2019.1698128
The impact of proline isomerization on antigen binding and the analytical profile of a trispecific anti-HIV antibody
Abstract
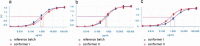
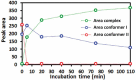
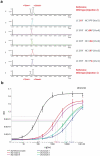
Proline cis-trans conformational isomerization is a mechanism that affects different types of protein functions and behaviors. Using analytical characterization, structural analysis, and molecular dynamics simulations, we studied the causes of an aberrant two-peak size-exclusion chromatography profile observed for a trispecific anti-HIV antibody. We found that proline isomerization in the tyrosine-proline-proline (YPP) motif in the heavy chain complementarity-determining region (CDR)3 domain of one of the antibody arms (10e8v4) was a component of this profile. The pH effect on the conformational equilibrium that led to these two populations was presumably caused by a histidine residue (H147) in the light chain that is in direct contact with the YPP motif. Finally, we demonstrated that, due to chemical equilibrium between the cis and trans proline conformers, the antigen-binding potency of the trispecific anti-HIV antibody was not significantly affected in spite of a potential structural clash of 10e8v4 YPtransPtrans conformers with the membrane-proximal ectodomain region epitope in the GP41 antigen. Altogether, these results reveal at mechanistic and molecular levels the effect of proline isomerization in the CDR on the antibody binding and analytical profiles, and support further development of the trispecific anti-HIV antibody.
Keywords: Proline isomerization; antibody conformers; chemical equilibrium; developability.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
