DPF is a cell-density sensing factor, with cell-autonomous and non-autonomous functions during Dictyostelium growth and development
- PMID: 31791330
- PMCID: PMC6889452
- DOI: 10.1186/s12915-019-0714-9
DPF is a cell-density sensing factor, with cell-autonomous and non-autonomous functions during Dictyostelium growth and development
Abstract
Background: Cellular functions can be regulated by cell-cell interactions that are influenced by extra-cellular, density-dependent signaling factors. Dictyostelium grow as individual cells in nutrient-rich sources, but, as nutrients become depleted, they initiate a multi-cell developmental program that is dependent upon a cell-density threshold. We hypothesized that novel secreted proteins may serve as density-sensing factors to promote multi-cell developmental fate decisions at a specific cell-density threshold, and use Dictyostelium in the identification of such a factor.
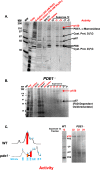
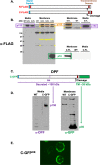
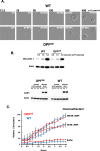
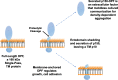
Results: We show that multi-cell developmental aggregation in Dictyostelium is lost upon minimal (2-fold) reduction in local cell density. Remarkably, developmental aggregation response at non-permissive cell densities is rescued by addition of conditioned media from high-density, developmentally competent cells. Using rescued aggregation of low-density cells as an assay, we purified a single, 150-kDa extra-cellular protein with density aggregation activity. MS/MS peptide sequence analysis identified the gene sequence, and cells that overexpress the full-length protein accumulate higher levels of a development promoting factor (DPF) activity than parental cells, allowing cells to aggregate at lower cell densities; cells deficient for this DPF gene lack density-dependent developmental aggregation activity and require higher cell density for cell aggregation compared to WT. Density aggregation activity co-purifies with tagged versions of DPF and tag-affinity-purified DPF possesses density aggregation activity. In mixed development with WT, cells that overexpress DPF preferentially localize at centers for multi-cell aggregation and define cell-fate choice during cytodifferentiation. Finally, we show that DPF is synthesized as a larger precursor, single-pass transmembrane protein, with the p150 fragment released by proteolytic cleavage and ectodomain shedding. The TM/cytoplasmic domain of DPF possesses cell-autonomous activity for cell-substratum adhesion and for cellular growth.
Conclusions: We have purified a novel secreted protein, DPF, that acts as a density-sensing factor for development and functions to define local collective thresholds for Dictyostelium development and to facilitate cell-cell communication and multi-cell formation. Regions of high DPF expression are enriched at centers for cell-cell signal-response, multi-cell formation, and cell-fate determination. Additionally, DPF has separate cell-autonomous functions for regulation of cellular adhesion and growth.
Keywords: Chemotaxis; Ecto-domain shedding; MS/MS peptide sequencing; Protein purification; Signaling.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







Similar articles
-
mTORC1/AMPK responses define a core gene set for developmental cell fate switching.BMC Biol. 2019 Jul 18;17(1):58. doi: 10.1186/s12915-019-0673-1. BMC Biol. 2019. PMID: 31319820 Free PMC article.
-
A cysteine-rich extracellular protein containing a PA14 domain mediates quorum sensing in Dictyostelium discoideum.Eukaryot Cell. 2005 Jun;4(6):991-8. doi: 10.1128/EC.4.6.991-998.2005. Eukaryot Cell. 2005. PMID: 15947191 Free PMC article.
-
LrrA, a novel leucine-rich repeat protein involved in cytoskeleton remodeling, is required for multicellular morphogenesis in Dictyostelium discoideum.Dev Biol. 2005 Sep 1;285(1):238-51. doi: 10.1016/j.ydbio.2005.05.045. Dev Biol. 2005. PMID: 16051212
-
PSF and CMF, autocrine factors that regulate gene expression during growth and early development of Dictyostelium.Experientia. 1995 Dec 18;51(12):1124-34. doi: 10.1007/BF01944730. Experientia. 1995. PMID: 8536800 Review.
-
Cell-cell signaling and adhesion in phagocytosis and early development of Dictyostelium.Int J Dev Biol. 2000;44(6):733-42. Int J Dev Biol. 2000. PMID: 11061438 Review.
Cited by
-
CRISPR/Cas9-based genome-wide screening of Dictyostelium.Sci Rep. 2022 Jul 2;12(1):11215. doi: 10.1038/s41598-022-15500-3. Sci Rep. 2022. PMID: 35780186 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

