Randomised controlled trial of the short-term effects of OROS-methylphenidate on ADHD symptoms and behavioural outcomes in young male prisoners with attention-deficit/hyperactivity disorder (CIAO-II)
- PMID: 31791384
- PMCID: PMC6889577
- DOI: 10.1186/s13063-019-3705-9
Randomised controlled trial of the short-term effects of OROS-methylphenidate on ADHD symptoms and behavioural outcomes in young male prisoners with attention-deficit/hyperactivity disorder (CIAO-II)
Abstract
Background: Attention-deficit/hyperactivity disorder (ADHD) is a highly prevalent disorder, seen in 20-30% of young adult prisoners. Pharmacoepidemiological studies, a small randomised controlled trial and open trial data of methylphenidate suggest clinically significant reductions in ADHD symptoms, emotional dysregulation, disruptive behaviour and increased engagement with educational activities. Yet, routine treatment of ADHD in offenders is not yet established clinical practice. There is continued uncertainty about the clinical response to methylphenidate (MPH), a first-line treatment for ADHD, in offenders, who often present with an array of complex mental health problems that may be better explained by states of inattentive, overactive, restless and impulsive behaviours. To address this problem, we will conduct an efficacy trial to establish the short-term effects of osmotic-controlled release oral delivery system (OROS)-methylphenidate (Concerta XL), an extended release formulation of MPH, on ADHD symptoms, emotional dysregulation and behaviour.
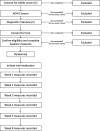
Methods: This study is a parallel-arm, randomised, placebo-controlled trial of OROS-MPH on ADHD symptoms, behaviour and functional outcomes in young male prisoners aged 16-25, meeting Diagnostic and Statistical Manual of Mental Disorders, fifth edition criteria for ADHD. Participants are randomised to 8 weeks of treatment with OROS-MPH or placebo, titrated over 5 weeks to balance ADHD symptom improvement against side effects. Two hundred participants will be recruited with a 1:1 ratio of drug to placebo. The primary outcome is change in level of ADHD symptoms after 8 weeks of trial medication.
Discussion: Potential benefits include improvement in ADHD symptoms, emotional dysregulation, attitudes towards violence and critical incidents and increased engagement with educational and rehabilitation programmes. Demonstrating the efficacy and safety of MPH on ADHD symptoms and associated impairments may provide the data needed to develop effective healthcare pathways for a significant group of young offenders. Establishing efficacy of MPH in this population will provide the foundation needed to establish long-term effectiveness studies with the potential for demonstrating significant reductions in criminal behaviour and improved health-economic outcomes.
Trial registration: ISRCTN registry, ISRCTN16827947, 31st May 2016; EudraCT number, 2015-004271-78, 31st May 2016. Last particpant last visit 6 June 2019. Data lock 27 August 2019.
Keywords: ADHD; Neurodevelopmental disorder; OROS-methylphenidate; Prison mental health; Trial.
Conflict of interest statement
King’s College London departmental research support account received payments for work by PA: consultancy to Shire, Eli Lilly and Novartis; educational/research awards from Shire, Lilly, Novartis, Vifor Pharma, GW Pharmaceuticals and QbTech; speaker at events sponsored by Shire, Lilly, Novartis, Flynn Pharma and Medicen. PA received personal honoaria for talking at events sponsored by Shire, Flynn Pharma and Medicen.
SY has received honoraria for consultation and/or educational talks in the last 5 years from Janssen, HB Pharma and/or Shire. She is author of the ADHD Child Evaluation (ACE) and ACE+ for adults and lead author of R&R2 for ADHD Youths and Adults.
In the last 3 years, SL has received personal fees from Janssen, Otsuka and Sunovion and research support from Janssen, all in connection with schizophrenia meetings and trials.
JS has worked with several pharmaceutical companies to seek to identify new or improved medications, but they do not have a relationship to the study reported here. This has included research grant support and consultancy payments to King’s College London and travelling and/or accommodation and/or conference expenses (including, in the past 3 years, from Martindale, Indivior, MundiPharma, Camurus/Braeburn).
The other authors declare that they have no competing interests.
Figures
Comment in
-
Methylphenidate for prison inmates with ADHD: yes or no?Br J Psychiatry. 2023 Jan;222(1):4-6. doi: 10.1192/bjp.2022.139. Br J Psychiatry. 2023. PMID: 36263739
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical