New Triazole NT-a9 Has Potent Antifungal Efficacy against Cryptococcus neoformans In Vitro and In Vivo
- PMID: 31791946
- PMCID: PMC6985734
- DOI: 10.1128/AAC.01628-19
New Triazole NT-a9 Has Potent Antifungal Efficacy against Cryptococcus neoformans In Vitro and In Vivo
Abstract
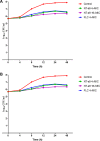
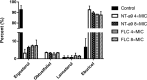
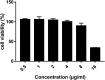
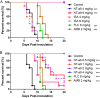
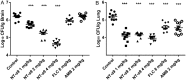
In the past decades, the incidence of cryptococcosis has increased dramatically, which poses a new threat to human health. However, only a few drugs are available for the treatment of cryptococcosis. Here, we described a leading compound, NT-a9, an analogue of isavuconazole, that showed strong antifungal activities in vitro and in vivo NT-a9 showed a wide range of activities against several pathogenic fungi in vitro, including Cryptococcus neoformans, Cryptococcus gattii, Candida albicans, Candida krusei, Candida tropicalis, Candida glabrata, and Candida parapsilosis, with MICs ranging from 0.002 to 1 μg/ml. In particular, NT-a9 exhibited excellent efficacy against C. neoformans, with a MIC as low as 0.002 μg/ml. NT-a9 treatment resulted in changes in the sterol contents in C. neoformans, similarly to fluconazole. In addition, NT-a9 possessed relatively low cytotoxicity and a high selectivity index. The in vivo efficacy of NT-a9 was assessed using a murine disseminated-cryptococcosis model. Mice were infected intravenously with 1.8 × 106 CFU of C. neoformans strain H99. In the survival study, NT-a9 significantly prolonged the survival times of mice compared with the survival times of the control group or the isavuconazole-, fluconazole-, or amphotericin B-treated groups. Of note, 4 and 8 mg/kg of body weight of NT-a9 rescued all the mice, with a survival rate of 100%. In the fungal-burden study, NT-a9 also significantly reduced the fungal burdens in brains and lungs, while fluconazole and amphotericin B only reduced the fungal burden in lungs. Taken together, these data suggested that NT-a9 is a promising antifungal candidate for the treatment of cryptococcosis infection.
Keywords: Cryptococcus neoformans; NT-a9; disseminated cryptococcosis; fungal burden; triazole compound.
Copyright © 2020 American Society for Microbiology.
Figures
References
-
- Netelenbos T, Massey E, de Wreede LC, Harding K, Hamblin A, Sekhar M, Li A, Ypma PF, Ball L, Zwaginga JJ, Stanworth SJ. 2019. The burden of invasive infections in neutropenic patients: incidence, outcomes, and use of granulocyte transfusions. Transfusion 59:160–168. doi:10.1111/trf.14994. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

