Nanog regulates Pou3f1 expression at the exit from pluripotency during gastrulation
- PMID: 31791948
- PMCID: PMC6899006
- DOI: 10.1242/bio.046367
Nanog regulates Pou3f1 expression at the exit from pluripotency during gastrulation
Abstract
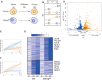
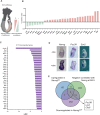
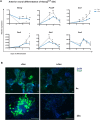
Pluripotency is regulated by a network of transcription factors that maintain early embryonic cells in an undifferentiated state while allowing them to proliferate. NANOG is a critical factor for maintaining pluripotency and its role in primordial germ cell differentiation has been well described. However, Nanog is expressed during gastrulation across all the posterior epiblast, and only later in development is its expression restricted to primordial germ cells. In this work, we unveiled a previously unknown mechanism by which Nanog specifically represses genes involved in anterior epiblast lineage. Analysis of transcriptional data from both embryonic stem cells and gastrulating mouse embryos revealed Pou3f1 expression to be negatively correlated with that of Nanog during the early stages of differentiation. We have functionally demonstrated Pou3f1 to be a direct target of NANOG by using a dual transgene system for the controlled expression of Nanog Use of Nanog null ES cells further demonstrated a role for Nanog in repressing a subset of anterior neural genes. Deletion of a NANOG binding site (BS) located nine kilobases downstream of the transcription start site of Pou3f1 revealed this BS to have a specific role in the regionalization of the expression of this gene in the embryo. Our results indicate an active role of Nanog inhibiting neural regulatory networks by repressing Pou3f1 at the onset of gastrulation.This article has an associated First Person interview with the joint first authors of the paper.
Keywords: Epiblast; Nanog; Pluripotency; Pou3f1; Regulatory genomics.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

