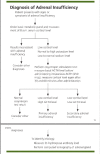
Nivolumab-induced adrenalitis
- PMID: 31791986
- PMCID: PMC6887423
- DOI: 10.1136/bcr-2019-231829
Nivolumab-induced adrenalitis
Abstract
Immune checkpoint inhibitors (ICIs) are an evolving class of drugs for the treatment of various cancers; for example, their use is recommended as a second-line chemotherapy for non-small cell lung cancer. With the expanding use of ICIs, we are discovering their unique side effects, called immune-related adverse events (irAEs), which can impair gastrointestinal, hepatic, dermatological, endocrine and other systems. Nivolumab is an ICI that blocks the human programmed death receptor-1 (PD-1) on T cells to prevent the interaction between the receptor, PD-1, and human programmed death ligand-1 expressed on tumour cells. Here, we report a case of a 65-year-old woman with recurrent lung adenocarcinoma who was treated with nivolumab and developed immune-related adrenalitis, which was managed with hydrocortisone and fludrocortisone. This case highlights the importance of understanding the irAEs of ICIs to allow prompt recognition and management of life-threatening complications of the treatment.
Keywords: adrenal disorders; chemotherapy; lung cancer (oncology); unwanted effects / adverse reactions.
© BMJ Publishing Group Limited 2019. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Yamada Y, Nishina T, Iwasa S, et al. . A phase I dose expansion trial of avelumab (MSB0010718C), an anti-PD-L1 antibody, in Japanese patients with advanced gastric cancer. Journal of Clinical Oncology 2015;33 10.1200/jco.2015.33.15_suppl.4047 - DOI
-
- Spira AI, Park K, Mazières J, et al. . Efficacy, safety and predictive biomarker results from a randomized phase II study comparing MPDL3280A vs docetaxel in 2L/3L NSCLC (poplar). Journal of Clinical Oncology 2015;33 10.1200/jco.2015.33.15_suppl.8010 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials