The Hybrid Histidine Kinase HrmK Is an Early-Acting Factor in the Hormogonium Gene Regulatory Network
- PMID: 31792014
- PMCID: PMC7015714
- DOI: 10.1128/JB.00675-19
The Hybrid Histidine Kinase HrmK Is an Early-Acting Factor in the Hormogonium Gene Regulatory Network
Abstract
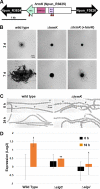
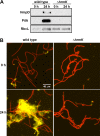
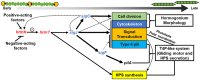
Filamentous, heterocyst-forming cyanobacteria belonging to taxonomic subsections IV and V are developmentally complex multicellular organisms capable of differentiating an array of cell and filament types, including motile hormogonia. Hormogonia exhibit gliding motility that facilitates dispersal, phototaxis, and the establishment of nitrogen-fixing symbioses. The gene regulatory network (GRN) governing hormogonium development involves a hierarchical sigma factor cascade, but the factors governing the activation of this cascade are currently undefined. Here, using a forward genetic approach, we identified hrmK, a gene encoding a putative hybrid histidine kinase that functions upstream of the sigma factor cascade. The deletion of hrmK produced nonmotile filaments that failed to display hormogonium morphology or accumulate hormogonium-specific proteins or polysaccharide. Targeted transcriptional analyses using reverse transcription-quantitative PCR (RT-qPCR) demonstrated that hormogonium-specific genes both within and outside the sigma factor cascade are drastically downregulated in the absence of hrmK and that hrmK may be subject to indirect, positive autoregulation via sigJ and sigC Orthologs of HrmK are ubiquitous among, and exclusive to, heterocyst-forming cyanobacteria. Collectively, these results indicate that hrmK functions upstream of the sigma factor cascade to initiate hormogonium development, likely by modulating the phosphorylation state of an unknown protein that may serve as the master regulator of hormogonium development in heterocyst-forming cyanobacteria.IMPORTANCE Filamentous cyanobacteria are morphologically complex, with several representative species amenable to routine genetic manipulation, making them excellent model organisms for the study of development. Furthermore, two of the developmental alternatives, nitrogen-fixing heterocysts and motile hormogonia, are essential to establish nitrogen-fixing symbioses with plant partners. These symbioses are integral to global nitrogen cycles and could be artificially recreated with crop plants to serve as biofertilizers, but to achieve this goal, detailed understanding and manipulation of the hormogonium and heterocyst gene regulatory networks may be necessary. Here, using the model organism Nostoc punctiforme, we identify a previously uncharacterized hybrid histidine kinase that is confined to heterocyst-forming cyanobacteria as the earliest known participant in hormogonium development.
Keywords: Nostoc punctiforme; cell motility; cyanobacteria; development; developmental biology; gliding motility; histidine kinase; hormogonia; two-component regulatory systems.
Copyright © 2020 American Society for Microbiology.
Figures


Similar articles
-
Hormogonium Development and Motility in Filamentous Cyanobacteria.Appl Environ Microbiol. 2023 Jun 28;89(6):e0039223. doi: 10.1128/aem.00392-23. Epub 2023 May 18. Appl Environ Microbiol. 2023. PMID: 37199640 Free PMC article. Review.
-
A Putative O-Linked β-N-Acetylglucosamine Transferase Is Essential for Hormogonium Development and Motility in the Filamentous Cyanobacterium Nostoc punctiforme.J Bacteriol. 2017 Apr 11;199(9):e00075-17. doi: 10.1128/JB.00075-17. Print 2017 May 1. J Bacteriol. 2017. PMID: 28242721 Free PMC article.
-
A Tripartite, Hierarchical Sigma Factor Cascade Promotes Hormogonium Development in the Filamentous Cyanobacterium Nostoc punctiforme.mSphere. 2019 May 1;4(3):e00231-19. doi: 10.1128/mSphere.00231-19. mSphere. 2019. PMID: 31043519 Free PMC article.
-
The primary transcriptome of hormogonia from a filamentous cyanobacterium defined by cappable-seq.Microbiology (Reading). 2021 Nov;167(11). doi: 10.1099/mic.0.001111. Microbiology (Reading). 2021. PMID: 34779764
-
Regulation of cellular differentiation in filamentous cyanobacteria in free-living and plant-associated symbiotic growth states.Microbiol Mol Biol Rev. 2002 Mar;66(1):94-121; table of contents. doi: 10.1128/MMBR.66.1.94-121.2002. Microbiol Mol Biol Rev. 2002. PMID: 11875129 Free PMC article. Review.
Cited by
-
Identification of a morphogene required for tapered filament termini in filamentous cyanobacteria.Microbiology (Reading). 2023 Nov;169(11):001416. doi: 10.1099/mic.0.001416. Microbiology (Reading). 2023. PMID: 37971486 Free PMC article.
-
Hormogonium Development and Motility in Filamentous Cyanobacteria.Appl Environ Microbiol. 2023 Jun 28;89(6):e0039223. doi: 10.1128/aem.00392-23. Epub 2023 May 18. Appl Environ Microbiol. 2023. PMID: 37199640 Free PMC article. Review.
-
The GGDEF protein Dgc2 suppresses both motility and biofilm formation in the filamentous cyanobacterium Leptolyngbya boryana.Microbiol Spectr. 2023 Sep 1;11(5):e0483722. doi: 10.1128/spectrum.04837-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 37655901 Free PMC article.
-
Transcriptomic and photosynthetic analyses of Synechocystis sp. PCC6803 and Chlorogloeopsis fritschii sp. PCC6912 exposed to an M-dwarf spectrum under an anoxic atmosphere.Front Plant Sci. 2024 Jan 18;14:1322052. doi: 10.3389/fpls.2023.1322052. eCollection 2023. Front Plant Sci. 2024. PMID: 38304456 Free PMC article.
-
EbsA is essential for both motility and biofilm formation in the filamentous cyanobacterium Nostoc punctiforme.Microbiology (Reading). 2024 Sep;170(9):001498. doi: 10.1099/mic.0.001498. Microbiology (Reading). 2024. PMID: 39287971 Free PMC article.
References
-
- Wong FC, Meeks JC. 2002. Establishment of a functional symbiosis between the cyanobacterium Nostoc punctiforme and the bryophyte Anthoceros punctatus requires genes involved in nitrogen control and initiation of heterocyst differentiation. Microbiology 148:315–323. doi:10.1099/00221287-148-1-315. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

