Molecular Imaging with Reporter Genes: Has Its Promise Been Delivered?
- PMID: 31792128
- PMCID: PMC12079160
- DOI: 10.2967/jnumed.118.220004
Molecular Imaging with Reporter Genes: Has Its Promise Been Delivered?
Abstract
The first reporter systems were developed in the early 1980s and were based on measuring the activity of an enzyme-as a surrogate measure of promoter-driven transcriptional activity-which is now known as a reporter gene system. The initial objective and application of reporter techniques was to analyze the activity of a specific promoter (namely, the expression of a gene that is under the regulation of the specific promoter that is linked to the reporter gene). This system allows visualization of specific promoter activity with great sensitivity. In general, there are 2 classes of reporter systems: constitutively expressed (always-on) reporter constructs used for cell tracking, and inducible reporter systems sensitive to endogenous signaling molecules and transcription factors that characterize specific tissues, tumors, or signaling pathways.This review traces the development of different reporter systems, using fluorescent and bioluminescent proteins as well as radionuclide-based reporter systems. The development and application of radionuclide-based reporter systems is the focus of this review. The question at the end of the review is whether the "promise" of reporter gene imaging has been realized. What is required for moving forward with radionuclide-based reporter systems, and what is required for successful translation to clinical applications?
© 2019 by the Society of Nuclear Medicine and Molecular Imaging.
Figures

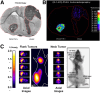
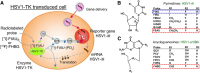

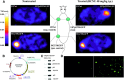
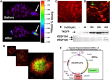

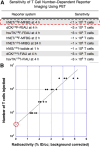

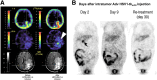
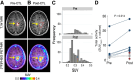

References
-
- Tjuvajev JG, Avril N, Oku T, et al. . Imaging herpes virus thymidine kinase gene transfer and expression by positron emission tomography. Cancer Res. 1998;58:4333–4341. - PubMed
-
- Contag CH, Spilman SD, Contag PR, et al. . Visualizing gene expression in living mammals using a bioluminescent reporter. Photochem Photobiol. 1997;66:523–531. - PubMed
-
- Pollok BA, Heim R. Using GFP in FRET-based applications. Trends Cell Biol. 1999;9:57–60. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
