Photoresponsive spiro-polymers generated in situ by C-H-activated polyspiroannulation
- PMID: 31792223
- PMCID: PMC6889291
- DOI: 10.1038/s41467-019-13308-w
Photoresponsive spiro-polymers generated in situ by C-H-activated polyspiroannulation
Abstract
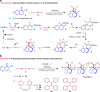
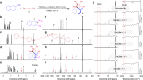
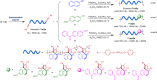
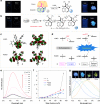
The development of facile and efficient polymerizations toward functional polymers with unique structures and attractive properties is of great academic and industrial significance. Here we develop a straightforward C-H-activated polyspiroannulation route to in situ generate photoresponsive spiro-polymers with complex structures. The palladium(II)-catalyzed stepwise polyspiroannulations of free naphthols and internal diynes proceed efficiently in dimethylsulfoxide at 120 °C without the constraint of apparent stoichiometric balance in monomers. A series of functional polymers with multisubstituted spiro-segments and absolute molecular weights of up to 39,000 are produced in high yields (up to 99%). The obtained spiro-polymers can be readily fabricated into different well-resolved fluorescent photopatterns with both turn-off and turn-on modes based on their photoinduced fluorescence change. Taking advantage of their photoresponsive refractive index, we successfully apply the polymer thin films in integrated silicon photonics techniques and achieve the permanent modification of resonance wavelengths of microring resonators by UV irradiation.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Wang, X. G. Azo Polymers: Synthesis, Functions and Applications (Springer, Berlin, 2017).
-
- Bertrand O, Gohy JF. Photo-responsive polymers: synthesis and applications. Polym. Chem. 2017;8:52–73. doi: 10.1039/C6PY01082B. - DOI
Publication types
LinkOut - more resources
Full Text Sources

