Plug-and-Play In Vitro Metastasis System toward Recapitulating the Metastatic Cascade
- PMID: 31792319
- PMCID: PMC6889311
- DOI: 10.1038/s41598-019-54711-z
Plug-and-Play In Vitro Metastasis System toward Recapitulating the Metastatic Cascade
Abstract
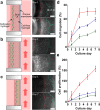
Microfluidic-based tumor models that mimic tumor culture environment have been developed to understand the cancer metastasis mechanism and discover effective antimetastatic drugs. These models successfully recapitulated key steps of metastatic cascades, yet still limited to few metastatic steps, operation difficulty, and small molecule absorption. In this study, we developed a metastasis system made of biocompatible and drug resistance plastics to recapitulate each metastasis stage in three-dimensional (3D) mono- and co-cultures formats, enabling the investigation of the metastatic responses of cancer cells (A549-GFP). The plug-and-play feature enhances the efficiency of the experimental setup and avoids initial culture failures. The results demonstrate that cancer cells tended to proliferate and migrate with circulating flow and intravasated across the porous membrane after a period of 3 d when they were treated with transforming growth factor-beta 1 (TGF-β1) or co-cultured with human pulmonary microvascular endothelial cells (HPMECs). The cells were also observed to detach and migrate into the circulating flow after a period of 20 d, indicating that they transformed into circulating tumor cells for the next metastasis stage. We envision this metastasis system can provide novel insights that would aid in fully understanding the entire mechanism of tumor invasion.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

