Drug-drug-gene interactions and adverse drug reactions
- PMID: 31792369
- PMCID: PMC7253354
- DOI: 10.1038/s41397-019-0122-0
Drug-drug-gene interactions and adverse drug reactions
Abstract
The economic and health burden caused by adverse drug reactions has increased dramatically in the last few years. This is likely to be mediated by increasing polypharmacy, which increases the likelihood for drug-drug interactions. Tools utilized by healthcare practitioners to flag potential adverse drug reactions secondary to drug-drug interactions ignore individual genetic variation, which has the potential to markedly alter the severity of these interactions. To date there have been limited published studies on impact of genetic variation on drug-drug interactions. In this review, we establish a detailed classification for pharmacokinetic drug-drug-gene interactions, and give examples from the literature that support this approach. The increasing availability of real-world drug outcome data linked to genetic bioresources is likely to enable the discovery of previously unrecognized, clinically important drug-drug-gene interactions.
Conflict of interest statement
There is no conflict of interest to disclose that could be perceived to bias our work. This work is a part of an entire PhD project which is supported by the main author’s sponsor for the purpose of completing his PhD degree. Authors and the sponsor have no conflict of interest to disclose.
Figures


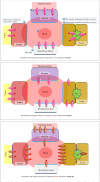
 to
to  = order of oral drug movement through different tissue types. Nonoral drug formulations bypass the effect of intestinal transporters.
= order of oral drug movement through different tissue types. Nonoral drug formulations bypass the effect of intestinal transporters.  /
/ = increased/decreased substrate drug plasma level is predicted as a result of impairment of this transporter due to LOF variants or inhibitors. The reverse is predicted with GOF variants or inducers. The presence of the two factors (i.e. LOF variant + an inhibitor or GOF variant + an inducer) is predicted to double the clinical impact with neutralizing or shifting the clinical effect when the preparator drug and genetic effect are opposed (phenoconversion interactions).
= increased/decreased substrate drug plasma level is predicted as a result of impairment of this transporter due to LOF variants or inhibitors. The reverse is predicted with GOF variants or inducers. The presence of the two factors (i.e. LOF variant + an inhibitor or GOF variant + an inducer) is predicted to double the clinical impact with neutralizing or shifting the clinical effect when the preparator drug and genetic effect are opposed (phenoconversion interactions).  = Apical membrane.
= Apical membrane.  = Basolateral membrane
= Basolateral membraneReferences
-
- Lazarou J, Pomernaz B, Corey P. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. Surv Anesthesiol. 1999;43:53–4. - PubMed
-
- MiDatabank Adverse Drug Reaction (ADR) reporting – 2016 update, SPS—Specialist Pharmacy Service – The first stop for professional medicines advice. 2017. Accessed 6 Sep 2017. https://www.sps.nhs.uk/articles/midatabank-adverse-drug-reaction-adr-rep....
-
- Exploring the costs of unsafe care in the NHS. frontier-economics. 2014. Accessed 11 Sep 2017. http://www.frontier-economics.com/documents/2014/10/exploring-the-costs-...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

