α-Synuclein strains target distinct brain regions and cell types
- PMID: 31792467
- PMCID: PMC6930851
- DOI: 10.1038/s41593-019-0541-x
α-Synuclein strains target distinct brain regions and cell types
Abstract
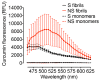
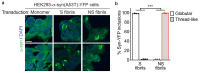
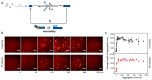
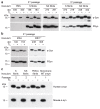
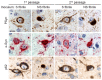
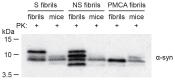
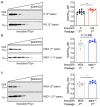
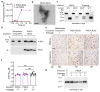
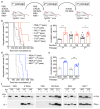
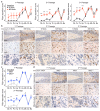
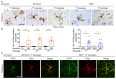
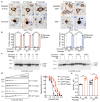
The clinical and pathological differences between synucleinopathies such as Parkinson's disease and multiple system atrophy have been postulated to stem from unique strains of α-synuclein aggregates, akin to what occurs in prion diseases. Here we demonstrate that inoculation of transgenic mice with different strains of recombinant or brain-derived α-synuclein aggregates produces clinically and pathologically distinct diseases. Strain-specific differences were observed in the signs of neurological illness, time to disease onset, morphology of cerebral α-synuclein deposits and the conformational properties of the induced aggregates. Moreover, different strains targeted distinct cellular populations and cell types within the brain, recapitulating the selective targeting observed among human synucleinopathies. Strain-specific clinical, pathological and biochemical differences were faithfully maintained after serial passaging, which implies that α-synuclein propagates via prion-like conformational templating. Thus, pathogenic α-synuclein exhibits key hallmarks of prion strains, which provides evidence that disease heterogeneity among the synucleinopathies is caused by distinct α-synuclein strains.
Conflict of interest statement
The authors declare no competing interests.
Figures




Comment in
-
α-Synuclein strains induce distinct diseases in mice.Nat Rev Neurol. 2020 Feb;16(2):66. doi: 10.1038/s41582-019-0306-x. Nat Rev Neurol. 2020. PMID: 31863058 No abstract available.
References
-
- Spillantini MG, Goedert M. The alpha-synucleinopathies: Parkinson's disease, dementia with Lewy bodies, and multiple system atrophy. Ann N Y Acad Sci. 2000;920:16–27. - PubMed
-
- Fujiwara H, et al. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–164. - PubMed
-
- Polymeropoulos MH, et al. Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

