Peptide-Catalyzed Fragment Couplings that Form Axially Chiral Non-C2 -Symmetric Biaryls
- PMID: 31793137
- PMCID: PMC7002259
- DOI: 10.1002/anie.201913563
Peptide-Catalyzed Fragment Couplings that Form Axially Chiral Non-C2 -Symmetric Biaryls
Abstract
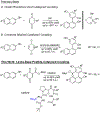
We have demonstrated that small, modular, tetrameric peptides featuring the Lewis-basic residue β-dimethylaminoalanine (Dmaa) are capable of atroposelectively coupling naphthols and ester-bearing quinones to yield non-C2 -symmetric BINOL-type scaffolds with good yields and enantioselectivity. The study culminates in the asymmetric synthesis of backbone-substituted scaffolds similar to 3,3'-disubstituted BINOLs, such as (R)-TRIP, with good (94:6 e.r.) to excellent (>99.9:0.1 e.r.) enantioselectivity after recrystallization, and a diastereoselective net arylation of the minimally modified nonsteroidal anti-inflammatory drug (NSAID) naproxen.
Keywords: atropisomerism; biaryl compounds; organocatalysis; peptides; quinones.
© 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
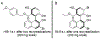


References
-
- Brussee J, Jansen ACA, Tetrahedron Lett. 1983, 24, 3261–3262;
- Lloyd-Williams P, Giralt E, Chem. Soc. Rev 2001, 30, 145–157;
- Hassan J, Sévignon M, Gozzi C, Schulz E, Lemaire M, Chem. Rev 2002, 102, 1359–1470; - PubMed
- Nelson TD, Crouch RD, Organic Reactions 2004, 63, 265–555;
- Wencel-Delord J, Panossian A, Leroux FR, Colobert F, Chem. Soc. Rev 2015, 44, 3418–3430; - PubMed
- Liao G, Zhou T, Yao Q-J, Shi B-F, Chem. Commun 2019, 55, 8514–8523. - PubMed
-
- Bringmann G, Breuning M, Tasler S, Synthesis 1999, 1999, 525–558;
- Bringmann G, Menche D, Acc. Chem. Res 2001, 34, 615–624; - PubMed
- Bringmann G, Breuning M, Pfeifer R-M, Schenk WA, Kamikawa K, Uemura M, J. Organomet. Chem 2002, 661, 31–47;
- Bringmann G, Tasler S, Pfeifer R-M, Breuning M, J. Organomet. Chem 2002, 661, 49–65;
- Bringmann G, Scharl H, Maksimenka K, Radacki K, Braunschweig H, Wich P, Schmuck C, Eur. J. Org. Chem 2006, 2006, 4349–4361.
-
- Gustafson JL, Lim D, Miller SJ, Science 2010, 328, 1251–1255; - PMC - PubMed
- Barrett KT, Miller SJ, J. Am. Chem. Soc 2013, 135, 2963–2966; - PMC - PubMed
- Diener ME, Metrano AJ, Kusano S, Miller SJ, J. Am. Chem. Soc 2015, 137, 12369–12377; - PMC - PubMed
- Metrano AJ, Abascal NC, Mercado BQ, Paulson EK, Miller SJ, Chem. Commun 2016, 52, 4816–4819. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

