The Effect of Food on the Single-Dose Bioavailability and Tolerability of the Highest Marketed Strength of Duloxetine
- PMID: 31793229
- PMCID: PMC7586977
- DOI: 10.1002/cpdd.759
The Effect of Food on the Single-Dose Bioavailability and Tolerability of the Highest Marketed Strength of Duloxetine
Abstract
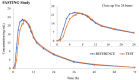
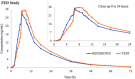
Duloxetine is a combined serotonin and norepinephrine reuptake inhibitor indicated in adults for the treatment of major depressive disorder, diabetic peripheral neuropathic pain, and generalized anxiety disorder. The aim of these studies was to evaluate the effect of food on the pharmacokinetics and safety of duloxetine 60-mg gastroresistant hard capsules following single-dose administration. The data were obtained from 2 phase 1 bioequivalence studies, 1 in a fasting state and the other under fed conditions. Both studies have shown that, when administered as a single dose in the same prandial state, the test and reference duloxetine treatments were bioequivalent and exhibited similar safety profiles. The mean fed and fasting pharmacokinetic parameters and drug-related adverse events from the 2 studies were compared in order to assess the effect of food on the duloxetine bioavailability and respectively, tolerability. Administration of duloxetine in fed conditions increased peak plasma concentration by up to 30% and delayed mean time to peak concentration by an average of 1.15 hours while having an insignificant effect on extent of absorption (area under the plasma concentration-time curve in fed state within ±6% as compared with fasting conditions). Even though peak plasma levels were substantially higher in the fed state, there was no negative impact on the drug's safety profile. Actually, administration with food resulted in a lower average number of adverse events per single dose exposure. The negligible variation in overall systemic exposure suggests that efficacy remains unchanged irrespective of administration conditions; however, a better tolerability of the 60-mg dose is expected when the drug is taken with food.
Keywords: HPLC-MS/MS; bioavailability; bioequivalence; duloxetine; pharmacokinetics; tolerability.
© 2019 The Authors. Clinical Pharmacology in Drug Development published by Wiley Periodicals, Inc. on behalf of American College of Clinical Pharmacology.
Figures
Similar articles
-
Duloxetine in elderly major depression disorder: effectiveness and drug plasma level evaluation.Hum Psychopharmacol. 2016 Sep;31(5):349-55. doi: 10.1002/hup.2544. Epub 2016 Jul 12. Hum Psychopharmacol. 2016. PMID: 27400882 Clinical Trial.
-
Dose proportionality and the effects of food on bioavailability of an immediate-release oxycodone hydrochloride tablet designed to discourage tampering and its relative bioavailability compared with a marketed oxycodone tablet under fed conditions: a single-dose, randomized, open-label, 5-way crossover study in healthy volunteers.Clin Ther. 2012 Jul;34(7):1601-12. doi: 10.1016/j.clinthera.2012.05.009. Epub 2012 Jun 19. Clin Ther. 2012. PMID: 22717418 Clinical Trial.
-
Efficacy and safety of duloxetine versus placebo in adolescents with juvenile fibromyalgia: results from a randomized controlled trial.Pediatr Rheumatol Online J. 2019 May 28;17(1):27. doi: 10.1186/s12969-019-0325-6. Pediatr Rheumatol Online J. 2019. PMID: 31138224 Free PMC article. Clinical Trial.
-
Effects of Food on Bioavailability of Analgesics; Resulting Dosage and Administration Recommendations.Pain Med. 2020 Nov 1;21(11):2877-2892. doi: 10.1093/pm/pnaa046. Pain Med. 2020. PMID: 32274507
-
Fast-Fed Variability: Insights into Drug Delivery, Molecular Manifestations, and Regulatory Aspects.Pharmaceutics. 2022 Aug 27;14(9):1807. doi: 10.3390/pharmaceutics14091807. Pharmaceutics. 2022. PMID: 36145555 Free PMC article. Review.
Cited by
-
Pharmacokinetics and Bioequivalence of a Generic Ticagrelor 90-mg Formulation Versus the Innovator Product in Healthy White Subjects Under Fasting Conditions.Clin Pharmacol Drug Dev. 2025 Jan;14(1):59-64. doi: 10.1002/cpdd.1471. Epub 2024 Sep 10. Clin Pharmacol Drug Dev. 2025. PMID: 39256193 Free PMC article. Clinical Trial.
-
Effectiveness of Duloxetine versus Other Therapeutic Modalities in Patients with Diabetic Neuropathic Pain: A Systematic Review and Meta-Analysis.Pharmaceuticals (Basel). 2024 Jun 28;17(7):856. doi: 10.3390/ph17070856. Pharmaceuticals (Basel). 2024. PMID: 39065707 Free PMC article. Review.
-
Antifungal and antibiofilm effect of duloxetine hydrochloride against Cryptococcus neoformans and Cryptococcus gattii.Folia Microbiol (Praha). 2024 Dec;69(6):1247-1254. doi: 10.1007/s12223-024-01164-1. Epub 2024 Apr 23. Folia Microbiol (Praha). 2024. PMID: 38652436
-
Duloxetine combined with intra-articular injection versus intra-articular injection alone for pain relief in knee osteoarthritis: a study protocol for a randomised controlled trial.BMJ Open. 2020 Oct 27;10(10):e036447. doi: 10.1136/bmjopen-2019-036447. BMJ Open. 2020. PMID: 33109641 Free PMC article.
References
-
- Fava M, Mallinckrodt CH, Detke MJ, Watkin JG, Wohlreich MM. The effect of duloxetine on painful physical symptoms in depressed patients: do improvements in these symptoms result in higher remission rates? J Clin Psychiatry. 2004;65(4):521‐530. - PubMed
-
- Detke MJ, Lu Y, Goldstein DJ, Hayes JR, Demitrack MA Duloxetine, 60 mg once daily, for major depressive disorder: a randomized double‐blind placebo‐controlled trial. J Clin Psychiatry. 2002;63(4):308‐315. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources