Polymer-mediated gene therapy: Recent advances and merging of delivery techniques
- PMID: 31793237
- PMCID: PMC7676468
- DOI: 10.1002/wnan.1598
Polymer-mediated gene therapy: Recent advances and merging of delivery techniques
Abstract
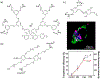
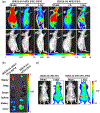
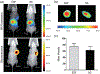
The ability to safely and precisely deliver genetic materials to target sites in complex biological environments is vital to the success of gene therapy. Numerous viral and nonviral vectors have been developed and evaluated for their safety and efficacy. This study will feature progress in synthetic polymers as nonviral vectors, which benefit from their chemical versatility, biocompatibility, and ability to carry both therapeutic cargo and targeting moieties. The combination of synthetic gene carrying constructs with advanced delivery techniques promises new therapeutic options for treating and curing genetic disorders. This article is characterized under: Therapeutic Approaches and Drug Discovery > Nanomedicine for Oncologic Disease Therapeutic Approaches and Drug Discovery > Emerging Technologies.
© 2019 Wiley Periodicals, Inc.
Conflict of interest statement
CONFLICT OF INTEREST
The authors have declared no conflicts of interest for this article.
Figures


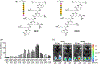

Similar articles
-
Emerging vectors and targeting methods for nonviral gene therapy.Expert Opin Emerg Drugs. 2006 Sep;11(3):541-57. doi: 10.1517/14728214.11.3.541. Expert Opin Emerg Drugs. 2006. PMID: 16939390 Review.
-
Vectors and strategies for nonviral cancer gene therapy.Expert Opin Biol Ther. 2016;16(4):443-61. doi: 10.1517/14712598.2016.1134480. Epub 2016 Jan 13. Expert Opin Biol Ther. 2016. PMID: 26761200 Review.
-
Structuring polymers for delivery of DNA-based therapeutics: updated insights.Crit Rev Ther Drug Carrier Syst. 2012;29(6):447-85. doi: 10.1615/critrevtherdrugcarriersyst.v29.i6.10. Crit Rev Ther Drug Carrier Syst. 2012. PMID: 23176056 Review.
-
Overcoming Gene-Delivery Hurdles: Physiological Considerations for Nonviral Vectors.Trends Biotechnol. 2016 Feb;34(2):91-105. doi: 10.1016/j.tibtech.2015.11.004. Epub 2015 Dec 23. Trends Biotechnol. 2016. PMID: 26727153 Free PMC article. Review.
-
Nonviral gene therapy: the promise of genes as pharmaceutical products.Hum Gene Ther. 1995 Sep;6(9):1129-44. doi: 10.1089/hum.1995.6.9-1129. Hum Gene Ther. 1995. PMID: 8527471 Review.
Cited by
-
Non-Viral Vectors for Delivery of Nucleic Acid Therapies for Cancer.BioTech (Basel). 2022 Mar 7;11(1):6. doi: 10.3390/biotech11010006. BioTech (Basel). 2022. PMID: 35822814 Free PMC article. Review.
-
Cellular uptake of modified mRNA occurs via caveolae-mediated endocytosis, yielding high protein expression in slow-dividing cells.Mol Ther Nucleic Acids. 2023 May 20;32:960-979. doi: 10.1016/j.omtn.2023.05.019. eCollection 2023 Jun 13. Mol Ther Nucleic Acids. 2023. PMID: 37305166 Free PMC article.
-
Cas-Based Systems for RNA Editing in Gene Therapy of Monogenic Diseases: In Vitro and in Vivo Application and Translational Potential.Front Cell Dev Biol. 2022 Jun 16;10:903812. doi: 10.3389/fcell.2022.903812. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35784464 Free PMC article. Review.
-
A New Strategy for Nucleic Acid Delivery and Protein Expression Using Biocompatible Nanohydrogels of Predefined Sizes.Pharmaceutics. 2023 Mar 16;15(3):961. doi: 10.3390/pharmaceutics15030961. Pharmaceutics. 2023. PMID: 36986821 Free PMC article.
-
Dendrimers for cancer immunotherapy: Avidity-based drug delivery vehicles for effective anti-tumor immune response.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2022 Mar;14(2):e1752. doi: 10.1002/wnan.1752. Epub 2021 Aug 19. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2022. PMID: 34414690 Free PMC article. Review.
References
-
- Altwaijry N, Somani S, Parkinson JA, Tate RJ, Keating P, Warzecha M, … Dufès C (2018). Regression of prostate tumors after intravenous administration of lactoferrin-bearing polypropylenimine dendriplexes encoding TNF-α, TRAIL, and interleukin-12. Drug Delivery, 25(1), 679–689. 10.1080/10717544.2018.1440666 - DOI - PMC - PubMed
-
- Anwer K, Kelly FJ, Chu C, Fewell JG, Lewis D, & Alvarez RD (2013). Phase I trial of a formulated IL-12 plasmid in combination with carboplatin and docetaxel chemotherapy in the treatment of platinum-sensitive recurrent ovarian cancer. Gynecologic Oncology, 131(1), 169–173. 10.1016/j.ygyno.2013.07.081 - DOI - PubMed
-
- Behr J (1997). The proton sponge: A trick to enter cells the viruses did not exploit. International Journal for Chemistry, 2(1), 34–36.
-
- Benner NL, McClellan RL, Turlington CR, Haabeth OAW, Waymouth RM, & Wender PA (2019). Oligo(serine ester) charge-altering releasable transporters: Organocatalytic ring-opening polymerization and their use for in vitro and in vivo mRNA delivery. Journal of the American Chemical Society, 141(21), 8416–8421. 10.1021/jacs.9b03154 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

