Beyond inflammasomes: emerging function of gasdermins during apoptosis and NETosis
- PMID: 31793683
- PMCID: PMC6960442
- DOI: 10.15252/embj.2019103397
Beyond inflammasomes: emerging function of gasdermins during apoptosis and NETosis
Abstract
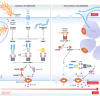
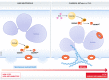
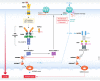
Programmed cell death is a key mechanism involved in several biological processes ranging from development and homeostasis to immunity, where it promotes the removal of stressed, damaged, malignant or infected cells. Abnormalities in the pathways leading to initiation of cell death or removal of dead cells are consequently associated with a range of human diseases including infections, autoinflammatory disease, neurodegenerative disease and cancer. Apoptosis, pyroptosis and NETosis are three well-studied modes of cell death that were traditionally believed to be independent of one another, but emerging evidence indicates that there is extensive cross-talk between them, and that all three pathways can converge onto the activation of the same cell death effector-the pore-forming protein Gasdermin D (GSDMD). In this review, we highlight recent advances in gasdermin research, with a particular focus on the role of gasdermins in pyroptosis, NETosis and apoptosis, as well as cell type-specific consequences of gasdermin activation. In addition, we discuss controversies surrounding a related gasdermin family protein, Gasdermin E (GSDME), in mediating pyroptosis and secondary necrosis following apoptosis, chemotherapy and inflammasome activation.
Keywords: NETosis; apoptosis; gasdermin; inflammasome; pyroptosis.
© 2019 The Authors.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J (2004) NALP3 forms an IL‐1beta‐processing inflammasome with increased activity in Muckle‐Wells autoinflammatory disorder. Immunity 20: 319–325 - PubMed
-
- Brennan MA, Cookson BT (2000) Salmonella induces macrophage death by caspase‐1‐dependent necrosis. Mol Microbiol 38: 31–40 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

