Metformin for prevention or delay of type 2 diabetes mellitus and its associated complications in persons at increased risk for the development of type 2 diabetes mellitus
- PMID: 31794067
- PMCID: PMC6889926
- DOI: 10.1002/14651858.CD008558.pub2
Metformin for prevention or delay of type 2 diabetes mellitus and its associated complications in persons at increased risk for the development of type 2 diabetes mellitus
Abstract
Background: The projected rise in the incidence of type 2 diabetes mellitus (T2DM) could develop into a substantial health problem worldwide. Whether metformin can prevent or delay T2DM and its complications in people with increased risk of developing T2DM is unknown.
Objectives: To assess the effects of metformin for the prevention or delay of T2DM and its associated complications in persons at increased risk for the T2DM.
Search methods: We searched the Cochrane Central Register of Controlled Trials, MEDLINE, Scopus, ClinicalTrials.gov, the World Health Organization (WHO) International Clinical Trials Registry Platform and the reference lists of systematic reviews, articles and health technology assessment reports. We asked investigators of the included trials for information about additional trials. The date of the last search of all databases was March 2019.
Selection criteria: We included randomised controlled trials (RCTs) with a duration of one year or more comparing metformin with any pharmacological glucose-lowering intervention, behaviour-changing intervention, placebo or standard care in people with impaired glucose tolerance, impaired fasting glucose, moderately elevated glycosylated haemoglobin A1c (HbA1c) or combinations of these.
Data collection and analysis: Two review authors read all abstracts and full-text articles and records, assessed risk of bias and extracted outcome data independently. We used a random-effects model to perform meta-analysis and calculated risk ratios (RRs) for dichotomous outcomes and mean differences (MDs) for continuous outcomes, using 95% confidence intervals (CIs) for effect estimates. We assessed the certainty of the evidence using GRADE.
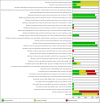
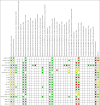
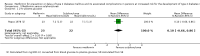
Main results: We included 20 RCTs randomising 6774 participants. One trial contributed 48% of all participants. The duration of intervention in the trials varied from one to five years. We judged none of the trials to be at low risk of bias in all 'Risk of bias' domains. Our main outcome measures were all-cause mortality, incidence of T2DM, serious adverse events (SAEs), cardiovascular mortality, non-fatal myocardial infarction or stroke, health-related quality of life and socioeconomic effects.The following comparisons mostly reported only a fraction of our main outcome set. Fifteen RCTs compared metformin with diet and exercise with or without placebo: all-cause mortality was 7/1353 versus 7/1480 (RR 1.11, 95% CI 0.41 to 3.01; P = 0.83; 2833 participants, 5 trials; very low-quality evidence); incidence of T2DM was 324/1751 versus 529/1881 participants (RR 0.50, 95% CI 0.38 to 0.65; P < 0.001; 3632 participants, 12 trials; moderate-quality evidence); the reporting of SAEs was insufficient and diverse and meta-analysis could not be performed (reported numbers were 4/118 versus 2/191; 309 participants; 4 trials; very low-quality evidence); cardiovascular mortality was 1/1073 versus 4/1082 (2416 participants; 2 trials; very low-quality evidence). One trial reported no clear difference in health-related quality of life after 3.2 years of follow-up (very low-quality evidence). Two trials estimated the direct medical costs (DMC) per participant for metformin varying from $220 to $1177 versus $61 to $184 in the comparator group (2416 participants; 2 trials; low-quality evidence). Eight RCTs compared metformin with intensive diet and exercise: all-cause mortality was 7/1278 versus 4/1272 (RR 1.61, 95% CI 0.50 to 5.23; P = 0.43; 2550 participants, 4 trials; very low-quality evidence); incidence of T2DM was 304/1455 versus 251/1505 (RR 0.80, 95% CI 0.47 to 1.37; P = 0.42; 2960 participants, 7 trials; moderate-quality evidence); the reporting of SAEs was sparse and meta-analysis could not be performed (one trial reported 1/44 in the metformin group versus 0/36 in the intensive exercise and diet group with SAEs). One trial reported that 1/1073 participants in the metformin group compared with 2/1079 participants in the comparator group died from cardiovascular causes. One trial reported that no participant died due to cardiovascular causes (very low-quality evidence). Two trials estimated the DMC per participant for metformin varying from $220 to $1177 versus $225 to $3628 in the comparator group (2400 participants; 2 trials; very low-quality evidence). Three RCTs compared metformin with acarbose: all-cause mortality was 1/44 versus 0/45 (89 participants; 1 trial; very low-quality evidence); incidence of T2DM was 12/147 versus 7/148 (RR 1.72, 95% CI 0.72 to 4.14; P = 0.22; 295 participants; 3 trials; low-quality evidence); SAEs were 1/51 versus 2/50 (101 participants; 1 trial; very low-quality evidence). Three RCTs compared metformin with thiazolidinediones: incidence of T2DM was 9/161 versus 9/159 (RR 0.99, 95% CI 0.41 to 2.40; P = 0.98; 320 participants; 3 trials; low-quality evidence). SAEs were 3/45 versus 0/41 (86 participants; 1 trial; very low-quality evidence). Three RCTs compared metformin plus intensive diet and exercise with identical intensive diet and exercise: all-cause mortality was 1/121 versus 1/120 participants (450 participants; 2 trials; very low-quality evidence); incidence of T2DM was 48/166 versus 53/166 (RR 0.55, 95% CI 0.10 to 2.92; P = 0.49; 332 participants; 2 trials; very low-quality evidence). One trial estimated the DMC of metformin plus intensive diet and exercise to be $270 per participant compared with $225 in the comparator group (94 participants; 1 trial; very-low quality evidence). One trial in 45 participants compared metformin with a sulphonylurea. The trial reported no patient-important outcomes. For all comparisons there were no data on non-fatal myocardial infarction, non-fatal stroke or microvascular complications. We identified 11 ongoing trials which potentially could provide data of interest for this review. These trials will add a total of 17,853 participants in future updates of this review.
Authors' conclusions: Metformin compared with placebo or diet and exercise reduced or delayed the risk of T2DM in people at increased risk for the development of T2DM (moderate-quality evidence). However, metformin compared to intensive diet and exercise did not reduce or delay the risk of T2DM (moderate-quality evidence). Likewise, the combination of metformin and intensive diet and exercise compared to intensive diet and exercise only neither showed an advantage or disadvantage regarding the development of T2DM (very low-quality evidence). Data on patient-important outcomes such as mortality, macrovascular and microvascular diabetic complications and health-related quality of life were sparse or missing.
Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Kasper S Madsen (KM): none known
Yuan Chi (YC): none known
Maria‐Inti Metzendorf (MM): none known
Bernd Richter (BR): none known
Bianca Hemmingsen (BH): none known
Figures
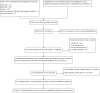














































Update of
References
References to studies included in this review
Alfawaz 2018 {published data only}
BIGPRO1 2009 {published data only}
-
- Charles MA, Morange P, Eschwege E, Andre P, Vague P, Juhan‐Vague I. Effect of weight change and metformin on fibrinolysis and the von willebrand factor in obese nondiabetic subjects: the BIGPRO1 Study. Biguanides and the prevention of the risk of obesity. Diabetes Care 1998. - PubMed
-
- Fontbonne A, Andre P, Eschwege E. BIGPRO (biguanides and the prevention of the risk of obesity): study design. A randomized trial of metformin versus placebo in the correction of the metabolic abnormalities associated with insulin resistance. Diabetes & Metabolism 1991;17(1 Pt 2):249‐54. [PUBMED: 1936485] - PubMed
-
- Fontbonne A, Charles MA, Juhan‐Vague I, Bard JM, Andre P, Isnard F, et al. Effects of 1‐year treatment with metformin on metabolic and cardiovascular risk factors in non‐diabetic upper‐body obese subjects with mild glucose anomalies: a post‐hoc analysis of the BIGPRO1 trial. Diabetes & Metabolism 2009;35(5):385‐91. - PubMed
-
- Fontbonne A, Charles MA, Juhan‐Vague I, Bard JM, Andre P, Isnard F, et al. The effect of metformin on the metabolic abnormalities associated with upper‐body fat distribution. Diabetes Care 1996;19(9):920‐6. - PubMed
Chen 2009 {published data only}
-
- Chen C, Ye ZP, Xie M, Xu XY. Intervene effect of Shenqi Jiangtang Capsule on type 2 diabetes developed from low glucose tolerance [参芪降糖胶囊在糖耐量低减人群向2型糖尿病发展中的干预作用]. Chinese Journal of Traditional Medical Science and Technology [中国中医药科技] 2009;16(1):58‐9. [DOI: 10.3969/j.issn.1005-7072.2009.01.026] - DOI
DPP/DPPOS 2002 {published data only}
-
- Diabetes Prevention Program Outcomes Study (DPPOS). https://clinicaltrials.gov/ct2/show/NCT00038727 Assessed 23rd of January 2017.
-
- Aroda VR, Christophi CA, Edelstein SL, Zhang P, Herman WH, Barrett‐Connor E, et al. Diabetes Prevention Program (DPP) Research Group. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: the Diabetes Prevention Program outcomes study 10‐year follow‐up. Journal Clinical Endocrinology and Metabolism 2015;100(4):1646‐53. - PMC - PubMed
-
- Carris NW, Cheng F, Kelly WN. The changing cost to prevent diabetes: a retrospective analysis of the Diabetes Prevention Program. Journal of the American Pharmaceutical Association 2017;57(6):717‐22. - PubMed
Fang 2004 {published data only}
-
- Fang YS, Li TY, Chen SY. Effect of medicine and non‐medicine intervention on the outcomes of patients with impaired glucose tolerance: 5‐year follow‐up [药物与非药物干预对糖耐量减低者结局的影响:5年随访]. Chinese Journal of Clinical Rehabilitation 1999;8(30):6562‐3.
IDPP‐1 2006 {published data only}
-
- NCT00279240. Life style modifications prevents type 2 diabetes in Asian Indians. https://clinicaltrials.gov/show/NCT00279240 (first posted 19 January 2006).
-
- Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V. The Indian diabetes prevention programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP‐1). Diabetologia 2006;49:289‐97. - PubMed
-
- Ramachandran A, Snehalatha C, Satyavani K, Sivasankari S, Vijay V. Metabolic syndrome does not increase the risk of conversion of impaired glucose tolerance to diabetes in Asian Indians‐‐Result of Indian diabetes prevention programme. Diabetes Research and Clinical Practice 2007;76(2):215‐8. [PUBMED: 16982107] - PubMed
-
- Ramachandran A, Snehalatha C, Yamuna A, Mary S, Ping Z. Cost‐effectiveness of the interventions in the primary prevention of diabetes among Asian Indians: within‐trial results of the Indian Diabetes Prevention Programme (IDPP). Diabetes Care 2007;30(10):2548‐52. - PubMed
Iqbal Hydrie 2012 {published data only}
Ji 2011 {published data only}
-
- Ji XZ, Guan CR, Du XM, Chen RQ. Effects of metformin on serum c‐reactive protein and insulin sensitivity index in prediabetic patients [二甲双胍对糖尿病前期患者血清C反应蛋白及胰岛素敏感指数的影响]. Chinese Journal of Clinical Pharmacology and Therapeutics [中国临床药理学与治疗学] 2011;16(8):920‐4.
Jin 2009 {published data only}
-
- Jin GX, Wang YM. Changes in insulin resistance and islet beta cell function in patients with impaired fasting glucose after intervention [空腹血糖受损人群干预后胰岛素抵抗与胰岛β细胞功能的改变]. Journal of Bengbu Medical College [蚌埠医学院学报] 2009;34(11):969‐71.
Li 1999 {published data only}
-
- Li C L, Pan CY, Lu JM. Effect of metformin on patients with impaired glucose tolerance. Diabetic Medicine 1999;16(6):477‐81. - PubMed
Li 2009 {published data only}
-
- Li JJ, Tao DB, Zhang XH, Yin GZ, Chen XY. Intervention of participants with composite impaired glucose regulation and obesity by Metformin [二甲双胍对肥胖合并混合糖调节受损的干预研究]. Central China Medical Journal [华中医学杂志] 2009;33(5):254‐6.
Liao 2012 {published data only}
-
- Liao XM. Clinical observation of acarbose and metformin in patients with impaired glucose tolerance [阿卡波糖和二甲双胍治疗糖耐量受损患者的临床观察]. Contemporary Medicine [当代医学] 2012;18(11):145‐6.
Lu 2002 {published data only}
-
- Lu JM, Pan CY, Tian H, Li CL, Yang GQ, Zhang XL, et al. Intervention of metformin and dietary fiber in the development of type 2 diabetes in people with low glucose tolerance [二甲双胍和食物纤维在糖耐量低减人群向2型糖尿病发展中的干预作用]. Chinese Journal of Diabetes [中国糖尿病杂志] 2002;‐(6):21‐4.
Lu 2010 {published data only}
-
- Lu YM. Effects comparison between pre‐diabetes lifestyle intervention and drug intervention [糖尿病前期生活方式干预与药物干预的效果比较]. Zhengzhou University Master Thesis. Zhengzhou University, 2010.
Maji 2005 {published data only}
-
- Maji D, Roy RU, Das S. Prevention of type 2 diabetes in the prediabetic population. Journal of the Indian Medical Association 2005;103(11):609‐11. - PubMed
Papoz 1978 {published data only}
-
- Eschwege E. French Study Group for Diabetes Epidemiology (A.F.E.D.I.A.). Short term effects (2 years) of oral treatment for diabetes in the borderline impairment of oral glucose tolerance test. Diabetologia 1974;10(363):Abstract 31.
-
- Papoz L, Job D, Eschwege E, Aboulker JP, Cubeau J, Pequignot G, et al. Effect of oral hypoglycaemic drugs on glucose tolerance and insulin secretion in borderline diabetic patients. Diabetologia 1978;15(5):373‐80. [PUBMED: 104897] - PubMed
PREVENT‐DM 2017 {published data only}
-
- NCT02088034. Intervention to promote weight loss in Latinas At‐risk for Diabetes. clinicaltrials.gov/ct2/show/NCT02088034 (first posted 14 March 2014).
-
- Perez A, Alos VA, Scanlan A, Maia CM, Davey A, Whitaker RC, et al. The rationale, design, and baseline characteristics of PREVENT‐DM: A community‐based comparative effectiveness trial of lifestyle intervention and metformin among Latinas with prediabetes. Contemporary Clinical Trials 2015;45(Pt B):320‐7. [PUBMED: 26597415] - PMC - PubMed
Wang 2009 {published data only}
-
- Wang BL, Zhang LL. Clinical observation of metformin in the treatment of prediabetes [二甲双胍治疗糖尿病前期的临床观察]. Collected papers of the 7th endocrine academic conference of Shanxi Medical Association [山西省医学会第七届内分泌学术会议论文集]. Tai Yuan [太原]: Shanxi Medical Association [山西省医学会], 2009:38‐9.
Zeng 2013 {published data only}
-
- Zeng ZH, Cai F, Qiu JF, Liu YQ, Zhou WZ, Weng LH. Observation on the clinical effect of different intervention methods in patients with impaired glucose regulation [糖调节受损人群不同干预方法的临床效果观察]. Health Research [健康研究] 2013;33(5):370‐2+5.
Zhao 2013 {published data only}
-
- Zhao XY, Sun JZ. Clinical observation of metformin in the treatment of obesity with prediabetes (46 cases report attached) [二甲双胍治疗肥胖合并糖尿病前期的临床观察(附46例报告)]. Journal of Hubei University of Science and Technology (Medical Sciences) [湖北科技学院学报(医学版)] 2013;27(1):27‐9.
References to studies excluded from this review
Acbay 1996 {published data only}
-
- Acbay O, Gundogdu S. The efficacy of metformin in the treatment of hypertensive subjects with impaired glucose tolerance: Its effects on insulin sensitivity, blood pressure, carbohydrate and lipid metabolism. Klinik Gelisim 1996;9(1):4019‐25.
Ballon 2011 {published data only}
-
- Ballon JS, Hamer RM, Catellier DJ, Stewart D, Lavange L, Golden L, et al. Metformin and impaired glucose tolerance in overweight persons with schizophrenia. Oxford University Press 2011;37:24.
Biarnés 2005 {published data only}
-
- Biarnés J, Fernández‐Real JM, Fernández‐Castañer M, Mar García M, Soler J, Ricart W. Differential regulation of insulin action and tumor necrosis factor alpha system activity by metformin. Metabolism 2005;54(2):235‐9. - PubMed
Bulcão 2007 {published data only}
-
- Bulcão C, Ribeiro‐Filho FF, Sañudo A, Roberta Ferreira SG. Effects of simvastatin and metformin on inflammation and insulin resistance in individuals with mild metabolic syndrome. American Journal of Cardiovascular Drugs 2007;7(3):219‐24. - PubMed
Caballero 2004 {published data only}
-
- Caballero AE, Delgado A, Aguilar‐Salinas CA, Herrera AN, Castillo JL, Cabrera T, et al. The differential effects of metformin on markers of endothelial activation and inflammation in subjects with impaired glucose tolerance: a placebo‐controlled, randomised clinical trial. Journal of Clinical Endocrinology and Metabolism 2004;89(8):3943‐8. - PubMed
Celik 2012 {published data only}
-
- Celik O, Acbay O, Celik O, Acbay O. Effects of metformin plus rosuvastatin on hyperandrogenism in polycystic ovary syndrome patients with hyperlipidaemia and impaired glucose tolerance. Journal of Endocrinological Investigation 2012;35(10):905‐10. - PubMed
Chazova 2006 {published data only}
-
- Chazova I, Almazov VA, Shlyakhto E. Moxonidine improves glycaemic control in mildly hypertensive, overweight patients: a comparison with metformin. Diabetes, Obesity & Metabolism 2006;8(4):456‐65. - PubMed
Chen 2013 {published data only}
-
- Chen Y, Su DD, Feng JG. Metformin combined with lifestyle guidance in the treatment of 34 patients with obesity prediabetes [二甲双胍联合生活方式指导治疗34例肥胖型糖尿病前期患者]. Journal of Practical Diabetology [实用糖尿病杂志] 2013;9(5):48‐9.
ChiCTR‐TRC‐09000548 {published data only}
-
- ChiCTR‐TRC‐09000548. A randomized controlled trial for investigating the protective effects of fenofibrate on prediabetes with hypertriglyceridemia. apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR‐TRC‐09000548 (accessed 30 March 2019).
CTRI/2013/02/003417 {published data only}
-
- CTRI/2013/02/003417. Restudy of the PURSE HIS Population for the prevalence of risk factors causing blood vessel damage to heart muscle, brain tissue or peripheral tissue and to give advice on diet, exercise and if necessary small dose of drug to prevent conversion of Pre‐Diabetes to Diabetes. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2013/02/003417 (accessed 30 March 2019).
Eguchi 2007 {published data only}
-
- Eguchi K. Comparison of the effects of pioglitazone and metformin on insulin resistance and hormonal markers in patients with impaired glucose tolerance and early diabetes. Hypertension Research 2007;30(1):23‐30. - PubMed
Esteghamati 2013 {published data only}
-
- Esteghamati A, Noshad S, Rabizadeh S, Ghavami M, Zandieh A, Nakhjavani M. Comparative effects of metformin and pioglitazone on omenti and leptin concentrations in patients with newly diagnosed diabetes: a randomised clinical trial. Regulatory Peptides 2013;182:1‐6. - PubMed
EUCTR‐000650‐21‐ES {published data only}
-
- EUCTR2018‐000650‐21‐ES. Clinical trial to evaluate biomarkers of vascular aging. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2018‐000650‐21‐ES (accessed 16 September 2019).
EUCTR2008‐004497‐40‐GB {published data only}
-
- EUCTR2008‐004497‐40‐GB. The effect of metformin on weight and cardiovascular risk markers in abdomenally obese subjects with impaired fasting glucose previously treated for 12 months with either rimonabant or placebo ‐ use of metformin following weight loss and improvement in CV risk. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2008‐004497‐40‐GB (accessed 20 March 2019).
Fleming 2002 {published data only}
-
- Fleming R, Hopkinson ZE, Michael Wallace A, Greer IA, Sattar N. Ovarian function and metabolic factors in women with oligomenorrhoea treated with metformin in a randomised double blind placebo‐controlled trial. Journal of Clinical Endocrinology and Metabolism 2002;87(2):569‐74. - PubMed
Flores‐Saenz 2003 {published data only}
-
- Flores‐Saenz JL, Trujillo‐Arriaga HM, Rivas‐Vilchis JF, Mendez‐Francisco JD, Alarcon‐Aguilar FJ, Roman‐Ramos R. Crossover and double blind study with metformin and rosiglitazone in impaired glucose tolerance subjects. Proceedings of the Western Pharmacology Society 2003;46:143‐7. - PubMed
Gómez‐Díaz 2012 {published data only}
-
- Gómez‐Díaz RA, Talavera JO, Pool EC, Ortiz‐Navarrete FV, Solórzano‐Santos F, Mondragón‐González R, et al. Metformin decreases plasma resistin concentrations in pediatric patients with impaired glucose tolerance: a placebo‐controlled randomised clinical trial. Metabolism 2012;61(9):1247‐55. - PubMed
Gore 2005 {published data only}
Gram 2011 {published data only}
Guardado‐Mendoza R 2018 {published data only}
-
- Guardado‐Mendoza R, Jimenez‐Ceja L, Farfan D, Alvarez‐Canales M, Salazar‐Lopez S, Montes De Oca M, et al. Diabetes prevention with lifestyle, linagliptin and metformin in patients with prediabetes: The PRELLIM project. Diabetologia 2018;61:S22‐3.
Haukeland 2008 {published data only}
-
- Haukeland JW, Konopski Z, Loeberg EM, Haaland TK, Volkmann HL, Raschpichler G. A randomised, placebo controlled trial with metformin in patients with NAFLD. Hepatology 2008;48(4):334‐9.
Ishida 2005 {published data only}
-
- Ishida W, Satoh J. Characteristic of metformin for treatment of impaired glucose tolerance. Nihon Rinsho 2005;63 Suppl 2:433‐7. - PubMed
Kato 2009 {published data only}
-
- Kato T, Sawai Y, Kanayama H, Taguchi H, Terabayashi T, Taki F, et al. Comparative study of low‐dose pioglitazone or metformin treatment in Japanese diabetic patients with metabolic syndrome. Experimental and Clinical Endocrinology & Diabetes 2009;117(10):593‐603. - PubMed
Kelly 2012 {published data only}
Kendall 2013 {published data only}
-
- Kendall D, Vail A, Amin R, Barrett T, Dimitri P, Ivison F, et al. Metformin in obese children and adolescents: the MOCA trial. Journal of Clinical Endocrinology and Metabolism 2013;98(1):322‐9. - PubMed
Kilic 2011 {published data only}
-
- Kilic S, Yilmaz N, Zulfikaroglu E, Erdogan G, Aydin M, Batioglu S. Inflammatory‐metabolic parameters in obese and non‐obese normoandrogenemic polycystic ovary syndrome during metformin and oral contraceptive treatment. Gynecological Endocrinology 2011;27(9):622‐9. - PubMed
Koev 2004 {published data only}
-
- Koev D, Koeva L. Treatment of subjects with obesity and impaired fasting glucose with metformin. Endokrinologya 2004;9(4):207‐13.
Krysiak 2012 {published data only}
-
- Krysiak R, Okopien B. Lymphocyte‐suppressing and systemic anti‐inflammatory effects of high‐dose metformin in simvastatin‐treated patients with impaired fasting glucose. Atherosclerosis 2012;225(2):403‐7. - PubMed
Lehtovirta 2001 {published data only}
-
- Lehtovirta M, Forsén B, Gullström M, Häggblom M, Eriksson JG, Taskinen MR, et al. Metabolic effects of metformin in patients with impaired glucose tolerance. Diabetic Medicine 2001;18(7):578‐83. - PubMed
Li 2009b {published data only}
-
- Li AM, Zhao J. Effect of renshen jianxin capsule for alleviating insulin resistance in patients with coronary heart disease and glucose tolerance impairment. Chinese Journal of Integrated Traditional & Western Medicine 2009;29(9):830‐3. - PubMed
LIMIT‐1 {published data only}
-
- Hertog HM, Vermeer SE, Achterberg S, Algra A, Kappelle LJ, Dippel DWJ, et al. Safety and feasibility of treatment with metformin in patients with TIA or minor ischaemic stroke and impaired glucose tolerance: a randomised open‐label phase II trial. Cerebrovascular Diseases 2009;17(Suppl 6):151.
Lu 2011 {published data only}
-
- Lu YH, Lu JM, Wang SY, Li CL, Zheng RP, Tian H, et al. Outcome of intensive integrated intervention in participants with impaired glucose regulation in China. Advances in Therapy 2011;28(6):511‐9. - PubMed
Malin 2013 {published data only}
-
- Malin SK, Braun B. Effect of metformin on substrate utilization after exercise training in adults with impaired glucose tolerance. Applied Physiology, Nutrition, & Metabolism 2013;38(4):427‐30. - PubMed
Medical letter {published data only}
-
- Anonymous. Metformin for prediabetes. JAMA 2017;317(11):1171. - PubMed
Morel 1999 {published data only}
-
- Morel Y, Golay A, Perneger T, Lehmann T, Vadas L, Pasik C, et al. Metformin treatment leads to an increase in basal, but not insulin‐stimulated, glucose disposal in obese patients with impaired glucose tolerance. Diabetic Medicine 1999;16(8):650‐5. - PubMed
-
- Morel Y, Lehmann T, Vadas L, Golay A. Effect of metformin on insulin‐resistance in obese patients with glucose‐intolerance. Schweizerische medizinische Wochenschrift 1996;126(Suppl 74/I):12.
NCT00108615 {published data only}
-
- NCT00108615. Effects of insulin sensitizers in subjects with impaired glucose tolerance. clinicaltrials.gov/ct2/show/NCT00108615 (first posted 5 March 2016).
NCT02338193 {published data only}
-
- NCT02338193. Dapagliflozin and metformin, alone and in combination, in overweight/obese prior GDM women (DAPA‐GDM). clinicaltrials.gov/ct2/show/NCT02338193 (first posted 14 January 2015).
NCT03258723 {published data only}
-
- NCT03258723. Diabetes prevention with lifestyle intervention and metformin escalation (LIME). clinicaltrials.gov/ct2/show/NCT03258723 (first posted 23 August 2017).
Pre‐DICTED {published data only}
-
- NCT03503942. The pre‐diabetes interventions and continued tracking to ease‐out diabetes (Pre‐DICTED) program. https://clinicaltrials.gov/ct2/show/NCT03503942 Accessed 16th. - PMC - PubMed
RESIST {published data only}
-
- Garnett SP, Gow M, Ho M, Baur LA, Noakes M, Woodhead HJ, et al. Optimal macronutrient content of the diet for adolescents with prediabetes; RESIST a randomised control trial. Journal of Clinical Endocrinology & Metabolism 2013;98(5):2116‐25. - PubMed
Retnakaran 2012 {published data only}
-
- Retnakaran R, Ye C, Hanley AJ, Harris SB, Zinman B. Discordant effects on central obesity, hepatic insulin resistance, and alanine aminotransferase of low‐dose metformin and thiazolidinedione combination therapy in patients with impaired glucose tolerance. Diabetes, Obesity & Metabolism 2012;14(1):91‐3. - PubMed
Rodríguez‐Moctezuma 2005 {published data only}
-
- Rodríguez‐Moctezuma JR, Robles‐López G, López‐Carmona JM, Gutiérrez‐Rosas MJ. Effects of metformin on the body composition in subjects with risk factors for type 2 diabetes. Diabetes, Obesity & Metabolism 2005;7(2):189‐92. - PubMed
Scheen 2009 {published data only}
-
- Scheen AJ, Tan MH, Betteridge DJ, Birkeland K, Schmitz O, Charbonnel B. Long‐term glycaemic effects of pioglitazone compared with placebo as add‐on treatment to metformin or sulphonylurea monotherapy in PROactive (PROactive 18). Diabetic Medicine 2009;26(12):1242‐9. - PubMed
Schuster 2004 {published data only}
-
- Schuster D, Gaillard T, Rhinesmith S, Habash D, Osei K. Impact of metformin on glucose metabolism in nondiabetic, obese African Americans: A placebo‐controlled, 24‐month randomised study. Diabetes Care 2004;27(11):2768‐9. - PubMed
SLCTR/2016/026 {unpublished data only}
-
- SLCTR/2016/026. Effects of metformin and lifestyle modifications on the progression of the atherosclerosis among individuals with impaired glucose tolerance. slctr.lk/trials/slctr‐2016‐026 (accessed 16 September 2019).
STOP‐NIDDM {published data only}
-
- Chiasson JL, Gomis R, Hanefeld M, Josse RG, Karasik A, Laakso M. The STOP‐NIDDM trial: An international study on the efficacy of an alpha‐ glucosidase inhibitor to prevent type 2 diabetes in a population with impaired glucose tolerance: Rationale, design, and preliminary screening data. Diabetes Care 1998;21(10):1720‐5. - PubMed
Stroup 2013 {published data only}
-
- Stroup S. Effect of metformin on weight in patients with schizophrenia with impaired fasting glucose. Neuropsychopharmacology. 2013; Vol. 38:S41.
Sultana 2012 {published data only}
-
- Sultana SS, Amin F, Rahman A, Afsana F. A comparative study with metformin and pioglitazone versus metformin alone in nonalcoholic fatty liver disease in newly detected glucose intolerant patients. Diabetologia 2012;55:S500.
UKPDS {published data only}
-
- Turner R, Rachman J, Holman R. UK Prospective Diabetes Study. Diabetes und Stoffwechsel 1996;5(3):78‐80.
Vitolins 2017 {published data only}
Wan 2010 {published data only}
-
- Wan Q, Wang F, Wang F, Guan Q, Liu Y, Wang C, et al. Regression to normoglycaemia by fenofibrate in pre‐diabetic subjects complicated with hypertriglyceridaemia: a prospective randomised controlled trial. Diabetic Medicine 2010;27(11):1312‐7. - PubMed
Zinman 2010 {published data only}
-
- Zinman B, Harris SB, Neuman J, Gerstein HC, Retnakaran RR, Raboud J, et al. Low‐dose combination therapy with rosiglitazone and metformin to prevent type 2 diabetes mellitus (CANOE trial): a double‐blind randomised controlled study. Lancet 2010;376(9735):103‐11. - PubMed
References to studies awaiting assessment
ChiCTR‐IPR‐17012309 {published data only}
-
- ChiCTR‐IPR‐17012309. The effect of metformin on the number and function of the circulating endothelial progenitor cells in prediabetic patients. www.chictr.org.cn/showproj.aspx?proj=21001 (accessed 5 April 2019).
EDIT 1997 {published data only}
-
- Anonymous. Early Diabetes Intervention Trial. https://www.dtu.ox.ac.uk/EDIT/ (accessed 15 March 2019).
-
- Citroën HA, Tunbridge FKE, Holman RR. Possible prevention of type 2 diabetes with acarbose or metformin over three years. Diabetologia 2000;43(Suppl. 1):A73.
-
- Holman RR, Blackwell L, Manley SE, Tucker L, Frighi V, Stratton IM. Results from the early diabetes intervention trial. Diabetes 2003;52(Suppl. 1):A16.
-
- Holman RR, Blackwell L, Stratton IM, Manley SE, Tucker L, Frighi V. Six‐years results from the Early Diabetes Intervention Trial. Diabetic Medicine 2003;20(Suppl. 2):15.
-
- Holman RR, North BV, Tunbridge FKE. Early Diabetes Intervention Trial. Diabetes 1997;46(Suppl 1):157A.
NCT02409238 {published data only}
-
- NCT02409238. Insulin resistance and mild cognitive impairment (IRMCI) study. clinicaltrials.gov/ct2/show/NCT02409238 (first posted 6 April 2015);‐:‐.
Polanco 2015 {published data only}
-
- Polanco MA, Barrientos R, Godinez SA, Sanchez S, Leanos R, Plascencia S. Preventing the development of type 2 diabetes in subjects at high risk in western Mexico, using metformin and changes in lifestyle, followed 10 years. Diabetes 2015;64:A687.
References to ongoing studies
CTRI/2017/09/009635 {published data only}
-
- CTRI/2017/09/009635. A study of life style modification with and without metformin in prediabetic subjects. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=16184 (accessed 5 April 2019).
ePRECIDE 2017 {published and unpublished data}
-
- Unpublished protocol. Provided by the investigators.
-
- EUCTR2013‐000418‐39‐AT. Early prevention of diabetes complications in people with hyperglycaemia in Europe ‐ e‐PREDICE. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2013‐000418‐39‐AT (accessed 8 April 2016).
-
- EudraCT 2013‐000418‐39. Early prevention of diabetes complications in people with hyperglycaemia in Europe. www.clinicaltrialsregister.eu/ctr‐search/search?query=2013‐000418‐39 (accessed 17 March 2016).
-
- NCT03222765. Prevention of microvascular complications in prediabetes e‐PREDICE Study (ePREDICE). clinicaltrials.gov/ct2/show/NCT03222765 (first posted 19 July 2017).
-
- ePRECIDE. The project. www.epredice.eu/en/the‐project (accessed 17 March 2016).
Espinoza 2019 {published data only}
-
- Espinoza SE, Musi N, Wang CP, Michalek J, Orsak B, Romo T, et al. Rationale and study design of a randomized clinical trial of metformin to prevent frailty in older adults with pre‐diabetes. Journals of Gerontology Biological Sciences and Medical Sciences March 2019; Vol. pii: glz078. - PMC - PubMed
-
- NCT02570672. Metformin for preventing frailty in high‐risk older adults. clinicaltrials.gov/ct2/show/NCT02570672 (first posted 7 October 2015).
Ji 2019 {published data only}
-
- Ji L, Sun N, Zhang Y, Zhang L, Shen S, Wang X, et al. Efficacy of metformin in preventing progression to diabetes in Chinese subjects with impaired glucose regulation: protocol for a multicenter, open‐label, randomized, controlled clinical study. Diabetes Obesity Metabolism September 2019;Epub ahead of print:No page numbers. - PubMed
-
- NCT03441750. Efficacy of metformin in preventing diabetes in China (ChinaDPP). clinicaltrials.gov/ct2/show/NCT03441750 (first posted 21 February 2018).
JPRN‐UMIN000018995 {published and unpublished data}
-
- JPRN‐UMIN000018995. Metformin therapy for East Asian women with recent gestational diabetes mellitus and glucose abnormalities: a multicenter, randomised, open‐label trial. upload.umin.ac.jp/cgi‐open‐bin/icdr_e/ctr_view.cgi?recptno=R000021900 (accessed 16 September 2019).
Nadeau 2014 {published and unpublished data}
-
- NCT01779362. RISE adult medication study (RISE Adult). clinicaltrials.gov/ct2/show/NCT01779362 (first posted 30 January 2013).
NCT01804049 {published data only}
-
- NCT01804049. Metformin and muscle in insulin‐resistant older veterans (M&M). clinicaltrials.gov/ct2/show/NCT01804049 (first posted 5 March 2013).
NCT02915198 {published and unpublished data}
-
- NCT02915198. Investigation of metformin in pre‐diabetes on atherosclerotic cardiovascular outcomes (VA‐IMPACT). clinicaltrials.gov/ct2/show/NCT02915198 (first posted 26 September 2016).
NCT02969798 {published data only}
-
- NCT02969798. Pre‐diabetes in subject with impaired fasting glucose (IFG) and impaired glucose tolerance (IGT). clinicaltrials.gov/show/NCT02969798 (first received 16 March 2016).
NCT03194009 {published data only}
-
- NCT03194009. Prudente; diabetes prevention via exercise, nutrition and treatment (PRuDENTE). clinicaltrials.gov/ct2/show/NCT03194009 (first posted 21 June 2017).
Rhee 2019 {published and unpublished data}
-
- NCT02981121. Hospital‐based diabetes prevention study in Korea. clinicaltrials.gov/ct2/show/NCT02981121 (first posted 2 December 2016).
Additional references
Abdul‐Ghani 2006
-
- Abdul‐Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes 2006;55(5):1430‐5. [PUBMED: 16644701] - PubMed
ADA 1997
-
- The Expert Committee on the diagnosis and classification of diabetes mellitus. Report of the Expert Committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1997;20(7):1183‐97. - PubMed
ADA 2003
-
- The Expert Committee on the diagnosis and classification of diabetes mellitus. Follow‐up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26(11):3160‐7. - PubMed
ADA 2008
-
- American Diabetes Association. Standards of medical care in diabetes ‐ 2008. Diabetes Care 2008;31(Suppl 1):S12‐54. [PUBMED: 18165335] - PubMed
ADA 2010
ADA 2015
-
- American Diabetes Association. Standards of medical care in diabetes‐2015. Diabetes Care 2015;38(Suppl 1):S1‐93. [PUBMED: 24357209] - PubMed
ADA 2017
-
- American Diabetes Association. Standards of medical care in diabetes‐2017. Diabetes Care 2017;40:(Suppl 1).
ADA 2019a
-
- American Diabetes Association. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes 2019. Diabetes Care January 2019;42 (Supplement 1):S90‐S102. - PubMed
AHFS 1999
-
- American Hospital Formulary Service (AHFS). Metformin hydrochloride. American Hospital Formulary Service Drug Information. Bethesda, USA: American Society of Health‐System Pharmacists, Inc. 1999; Vol. 2755–63.
Altman 2003
Bell 2013
-
- Bell ML, McKenzie JE. Designing psycho‐oncology randomised trials and cluster randomised trials: variance components and intra‐cluster correlation of commonly used psychosocial measures. Psychooncology 2013;22:1738‐47. - PubMed
Borenstein 2017a
-
- Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta‐analysis: I² is not an absolute measure of heterogeneity. Research Synthesis Methods 2017;8(1):5‐18. - PubMed
Borenstein 2017b
-
- Borenstein M. Prediction intervals. www.meta‐analysis.com/prediction (accessed 3 July 2017).
Boutron 2014
-
- Boutron I, Altman DG, Hopewell S, Vera‐Badillo F, Tannock I, Ravaud P. Impact of spin in the abstracts of articles reporting results of randomized controlled trials in the field of cancer: the SPIIN randomized controlled trial. Journal of Clinical Oncology 2014;32:4120‐6. - PubMed
Centers for Disease Control and Prevention 2015
-
- http://www.cdc.gov/diabetes/data/statistics/2014statisticsreport.html. Assessed October 2015.
Cheng 2006
-
- Cheng C, Kushner H, Falkner BE. The utility of fasting glucose for detection of prediabetes. Metabolism: Clinical and Experimental 2006;55(4):434‐8. [PUBMED: 16546472] - PubMed
Cho 2015
Corbett 2014
-
- Corbett MS, Higgins JP, Woolacott NF. Assessing baseline imbalance in randomised trials: implications for the Cochrane risk of bias tool. Research Synthesis Methods 2014;5(1):79‐85. - PubMed
Corey 2007
-
- Corey EJ, Czakó B, Kürti L. Molecules and Medicine. Hoboken, NJ: Wiley, 2007. [978‐0‐470‐22749‐7]
Deeks 2017
-
- Deeks JJ, Higgins JP, Altman DG (editors), on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
DeFronzo 1989
-
- DeFronzo RA, Ferrannini E, Simonson DC. Fasting hyperglycemia in non‐insulin‐dependent diabetes mellitus: contributions of excessive hepatic glucose production and impaired tissue glucose uptake. Metabolism: Clinical and Experimental 1989;38(4):387‐95. [PUBMED: 2657323] - PubMed
DeFronzo 1999
-
- DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Annals of Internal Medicine 1999;131(4):281‐303. - PubMed
Diabetes Prevention Program 2002
Diabetes Prevention Program FU 2009
-
- Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, et al. Diabetes Prevention Program Research Group. 10‐year follow‐up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009 Nov 14;374(9702):1677‐86. [DOI: 10.1016/S0140‐6736(09)61457‐4; PUBMED: PMID: 19878986] - PMC - PubMed
diabetes.co.uk 2019a
-
- Diabetes UK. Blood Sugar Converter. https://www.diabetes.co.uk/blood‐sugar‐converter.html Accessed 30 March 2019.
diabetes.co.uk 2019b
-
- Diabetes UK. Convert Whole Blood Results to Plasma Readings. https://www.diabetes.co.uk/whole‐blood‐readings‐to‐plasma‐converter.html Accessed 30 March 2019.
DPP 2002
Duca 2015
Dunkley 2014
-
- Dunkley AJ, Bodicoat DH, Greaves CJ, Russell C, Yates T, Davies MJ, et al. Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: a systematic review and meta‐analysis. Diabetes Care 2014;37(4):922‐33. [PUBMED: 24652723] - PubMed
FDA 1994
-
- FDA. FDA Approved Drug Products. https://www.accessdata.fda.gov/drugsatfda_docs/nda/pre96/020357Orig1s000.... Assessed 20th September 2019.
Finnish Diabetes Prevention Study Group 2001
-
- Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne‐Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. New England Journal of Medicine 2001;344(18):1343‐50. [PUBMED: 11333990] - PubMed
Gosmanov 2014
-
- Gosmanov AR, Wan J. Low positive predictive value of hemoglobin A1c for diagnosis of prediabetes in clinical practice. American Journal of the Medical Sciences 2014;348(3):191‐4. [PUBMED: 24556928] - PubMed
Haw 2017
Higgins 2002
-
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011c
-
- Higgins JP, Deeks JJ, Altman DG, editor(s) on behalf of the Cochrane Statistical Methods Group. Chapter 16: Special topics in statistics. In: Higgins JT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA (editors) on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017), Cochrane, 2017. Available from training.cochrane.org/handbook.
Hróbjartsson 2013
-
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201‐11. - PMC - PubMed
Huang 2016
ICH 1997
-
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice //1997 CFR & ICH Guidelines. PA 19063‐2043. USA: Barnett International/PAREXEL, 1997.
IDF 2013
-
- International Diabetes Federation. International Diabetes Federation. IDF Diabetes Atlas, 6th ed.Brussels, Belgium. International Diabetes Federation, 2013.
IEC 2009
Inzucchi 2012
-
- Inzucchi SE. Clinical practice. Diagnosis of diabetes. New England Journal of Medicine 2012;367(6):542‐50. [PUBMED: 22873534] - PubMed
Jones 2015
Kalantar‐Zadeh 2013
-
- Kalantar‐Zadeh K, Uppot RN, Lewandrowski KB. Case records of the Massachusetts General Hospital. Case 23‐2013. A 54‐year‐old woman with abdominal pain, vomiting, and confusion. New England Journal of Medicine 2013;369(4):374‐82. [PUBMED: 23841704] - PubMed
Kirkham 2010
Kreisberg 1980
-
- Kreisberg RA. Lactate homeostasis and lactic acidosis. Annals of Internal Medicine 1980;92(2 Pt 1):227‐37. [PUBMED: 6766289] - PubMed
Liberati 2009
Lily 2009
Lundh 2017
Mathieu 2009
-
- Mathieu S, Boutron I, Moher D, Altman DG, Ravaud P. Comparison of registered and published primary outcomes in randomized controlled trials. JAMA 2009;302:977‐84. - PubMed
Meader 2014
Moelands 2018
-
- Moelands SV, Lucassen PL, Akkermans RP, Grauw WJ, Laar FA. Alpha‐glucosidase inhibitors for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2018;12:CD005061. - PMC - PubMed
Moin 2018
Morris 2013
-
- Morris DH, Khunti K, Achana F, Srinivasan B, Gray LJ, Davies MJ, et al. Progression rates from HbA1c 6.0‐6.4% and other prediabetes definitions to type 2 diabetes: a meta‐analysis. Diabetologia 2013;56(7):1489‐93. [PUBMED: 23584433] - PubMed
NDDG 1979
-
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039‐57. - PubMed
Pang 2018
-
- Pang B, Zhao LH, Li XL, Song J, Li QW, Liao X, et al. Different intervention strategies for preventing type 2 diabetes mellitus in China: A systematic review and network meta‐analysis of randomized controlled trials. Diabetes Obesity Metabolism 2018;20(3):718‐22. - PubMed
Riley 2011
-
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ 2011;342:d549. - PubMed
Salpeter 2008
-
- Salpeter SR, Buckley NS, Kahn JA, Salpeter EE. Meta‐analysis: metformin treatment in persons at risk for diabetes mellitus. American Journal of Medicine 2008;121(2):149‐57. - PubMed
Salpeter 2010
Schousboe 2012
-
- Schousboe K, Fassi D, Secher EL, Elming H, Rasmussen K, Hornum M. Treatment of metformin‐associated lactate acidosis by haemodialysis [Behandling af metforminassocieret laktatacidose med haemodialyse]. Ugeskrift for Laeger 2012;174(23):1604‐6. [PUBMED: 22673381] - PubMed
Selvin 2011
Sterne 2011
-
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. - PubMed
Sterne 2017
-
- Sterne JA, Egger M, Moher D, Boutron I (editors). Chapter 10: Addressing reporting biases. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
UKPDS 1998
-
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood‐glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352(9131):854‐65. - PubMed
Van de Laar 2006
Viera 2011
-
- Viera AJ. Predisease: when does it make sense?. Epidemiologic Reviews 2011;33:122‐34. - PubMed
Vist 2008
-
- Vist GE, Bryant D, Somerville L, Birminghem T, Oxman AD. Outcomes of patients who participate in randomized controlled trials compared to similar patients receiving similar interventions who do not participate. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.MR000009.pub4; PUBMED: 18677782] - DOI - PMC - PubMed
WHO 1998
-
- Alberti KM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its compliactions. Part I: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Medicine 1998;15:539‐53. - PubMed
WHO 1999
-
- World Health Organization: definition, diagnosis, classification of diabetes mellitus, its complications. Part 1: diagnosis and classification of diabetes mellitus. Report of a World Health Organization Consultation. Geneva: World Health Organization, 1999:1‐59.
WHO/IDF 2006
-
- World Health Organization/ International Diabetes Federation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation. http://www.idf.org/webdata/docs/WHO_IDF_definition_diagnosis_of_diabetes... 2006; Vol. Assessed October 2015.
Witters 2001
Wood 2008
References to other published versions of this review
Lü 2010
-
- Lü Q, Ke LQ, Tong N, Cao L, Wu T, Zhang J. Metformin for people with impaired glucose tolerance or impaired fasting blood glucose. Cochrane Database of Systematic Reviews 2010, Issue 6. [DOI: 10.1002/14651858.CD008558] - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

