Targeting the Oncogenic p53 Mutants in Colorectal Cancer and Other Solid Tumors
- PMID: 31795192
- PMCID: PMC6929124
- DOI: 10.3390/ijms20235999
Targeting the Oncogenic p53 Mutants in Colorectal Cancer and Other Solid Tumors
Abstract
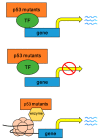
Colorectal cancer (CRC) is a kind of solid tumor and the third most common cancer type in the world. It is a heterogeneous disease characterized by genetic and epigenetic aberrations. The TP53 mutation is the key step driving the transition from adenoma to adenocarcinoma. The functional roles of TP53 mutation in tumor development have been comprehensively investigated. In CRC, TP53 mutation was associated with poor prognosis and chemoresistance. A gain of function (GOF) of p53 mutants promotes cell proliferation, migration and invasion through multiple mechanisms. Restoring wild type p53 function, depleting p53 mutants, or intervention by targeting the oncogenic downstreams provides potential therapeutic strategies. In this review, we comprehensively summarize the GOF of p53 mutants in CRC progression as well as in some other solid tumors, and discuss the current strategies targeting p53 mutants in malignancies.
Keywords: TP53; colorectal cancer; p53 mutants; solid tumor.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures

References
-
- Bhandari A., Woodhouse M., Gupta S. Colorectal cancer is a leading cause of cancer incidence and mortality among adults younger than 50 years in the USA: A SEER-based analysis with comparison to other young-onset cancers. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 2017;65:311–315. doi: 10.1136/jim-2016-000229. - DOI - PMC - PubMed
-
- Otori K., Konishi M., Sugiyama K., Hasebe T., Shimoda T., Kikuchi-Yanoshita R., Mukai K., Fukushima S., Miyaki M., Esumi H. Infrequent somatic mutation of the adenomatous polyposis coli gene in aberrant crypt foci of human colon tissue. Cancer. 1998;83:896–900. doi: 10.1002/(SICI)1097-0142(19980901)83:5<896::AID-CNCR14>3.0.CO;2-Q. - DOI - PubMed
-
- Miyaki M., Konishi M., Kikuchi-Yanoshita R., Enomoto M., Igari T., Tanaka K., Muraoka M., Takahashi H., Amada Y., Fukayama M., et al. Characteristics of somatic mutation of the adenomatous polyposis coli gene in colorectal tumors. Cancer Res. 1994;54:3011–3020. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

