Growth Plate Chondrocytes: Skeletal Development, Growth and Beyond
- PMID: 31795305
- PMCID: PMC6929081
- DOI: 10.3390/ijms20236009
Growth Plate Chondrocytes: Skeletal Development, Growth and Beyond
Abstract
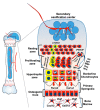
Growth plate chondrocytes play central roles in the proper development and growth of endochondral bones. Particularly, a population of chondrocytes in the resting zone expressing parathyroid hormone-related protein (PTHrP) is now recognized as skeletal stem cells, defined by their ability to undergo self-renewal and clonally give rise to columnar chondrocytes in the postnatal growth plate. These chondrocytes also possess the ability to differentiate into a multitude of cell types including osteoblasts and bone marrow stromal cells during skeletal development. Using single-cell transcriptomic approaches and in vivo lineage tracing technology, it is now possible to further elucidate their molecular properties and cellular fate changes. By discovering the fundamental molecular characteristics of these cells, it may be possible to harness their functional characteristics for skeletal growth and regeneration. Here, we discuss our current understanding of the molecular signatures defining growth plate chondrocytes.
Keywords: chondrocyte; endochondral ossification; growth plate; hedgehog; osteoblast; parathyroid hormone-related protein; regeneration; skeleton.
Conflict of interest statement
The authors declare no conflict of interest. Authors declare that there are no competing financial and/or non-financial interests regarding the publication of this paper. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Hinchliffe J.R., Gumpel-Pinot M. Control of maintenance and anteroposterior skeletal differentiation of the anterior mesenchyme of the chick wing bud by its posterior margin (the ZPA) J. Embryol. Exp. Morphol. 1981;62:63–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

