PP2Ac Modulates AMPK-Mediated Induction of Autophagy in Mycobacterium bovis-Infected Macrophages
- PMID: 31795474
- PMCID: PMC6928646
- DOI: 10.3390/ijms20236030
PP2Ac Modulates AMPK-Mediated Induction of Autophagy in Mycobacterium bovis-Infected Macrophages
Abstract
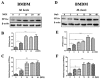
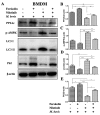
Mycobacterium bovis (M. bovis) is the causative agent of bovine tuberculosis in cattle population across the world. Human beings are at equal risk of developing tuberculosis beside a wide range of M. bovis infections in animal species. Autophagic sequestration and degradation of intracellular pathogens is a major innate immune defense mechanism adopted by host cells for the control of intracellular infections. It has been reported previously that the catalytic subunit of protein phosphatase 2A (PP2Ac) is crucial for regulating AMP-activated protein kinase (AMPK)-mediated autophagic signaling pathways, yet its role in tuberculosis is still unclear. Here, we demonstrated that M. bovis infection increased PP2Ac expression in murine macrophages, while nilotinib a tyrosine kinase inhibitor (TKI) significantly suppressed PP2Ac expression. In addition, we observed that TKI-induced AMPK activation was dependent on PP2Ac regulation, indicating the contributory role of PP2Ac towards autophagy induction. Furthermore, we found that the activation of AMPK signaling is vital for the regulating autophagy during M. bovis infection. Finally, the transient inhibition of PP2Ac expression enhanced the inhibitory effect of TKI-nilotinib on intracellular survival and multiplication of M. bovis in macrophages by regulating the host's immune responses. Based on these observations, we suggest that PP2Ac should be exploited as a promising molecular target to intervene in host-pathogen interactions for the development of new therapeutic strategies towards the control of M. bovis infections in humans and animals.
Keywords: AMPK; Mycobacterium bovis (M. bovis), PP2Ac; TKI; autophagy; macrophages.
Conflict of interest statement
All authors read has approved the final version of this manuscript. The authors declare that neither they nor their respective institutions have any competing conflict of interests.
Figures



References
MeSH terms
Substances
Grants and funding
- 31572487, 31873005/National Natural Science Foundation of China
- 2017YFD0500901/National Key Research and Development Program
- CARS-36/China Agriculture Research System
- 2013DFG32500/the MoSTRCUK international cooperation project
- GDW20151100036, GDW20161100071/the High-end Foreign Experts Recruitment Program
LinkOut - more resources
Full Text Sources
Medical

