Thermosensitive Liposome-Mediated Drug Delivery in Chemotherapy: Mathematical Modelling for Spatio-temporal Drug Distribution and Model-Based Optimisation
- PMID: 31795486
- PMCID: PMC6955700
- DOI: 10.3390/pharmaceutics11120637
Thermosensitive Liposome-Mediated Drug Delivery in Chemotherapy: Mathematical Modelling for Spatio-temporal Drug Distribution and Model-Based Optimisation
Abstract
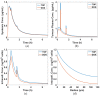
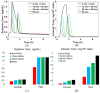
Thermosensitive liposome-mediated drug delivery has shown promising results in terms of improved therapeutic efficacy and reduced side effects compared to conventional chemotherapeutics. In order to facilitate our understanding of the transport mechanisms and their complex interplays in the drug delivery process, computational models have been developed to simulate the multiple steps involved in liposomal drug delivery to solid tumours. In this study we employ a multicompartmental model for drug-loaded thermosensitive liposomes, with an aim to identify the key transport parameters in determining therapeutic dosing and outcomes. The computational model allows us to not only examine the temporal and spatial variations of drug concentrations in the different compartments by utilising the tumour cord concept, but also assess the therapeutic efficacy and toxicity. In addition, the influences of key factors on systemic plasma concentration and intracellular concentration of the active drug are investigated; these include different chemotherapy drugs, release rate constants and heating duration. Our results show complex relationships between these factors and the predicted therapeutic outcome, making it difficult to identify the "best" parameter set. To overcome this challenge, a model-based optimisation method is proposed in an attempt to find a set of release rate constants and heating duration that can maximise intracellular drug concentration while minimising systemic drug concentration. Optimisation results reveal that under the operating conditions and ranges examined, the best outcome would be achieved with a low drug release rate at physiological temperature, combined with a moderate to high release rate at mild hyperthermia and 1 h heating after injection.
Keywords: drug delivery; mathematical model; multi-compartmental model; optimisation; thermosensitive liposome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

