Safety of medication use during pregnancy in mainland China: based on a national health insurance database in 2015
- PMID: 31795963
- PMCID: PMC6892234
- DOI: 10.1186/s12884-019-2622-y
Safety of medication use during pregnancy in mainland China: based on a national health insurance database in 2015
Abstract
Background: Medication safety during pregnancy has drawn global attention, little of which has been reported about the Chinese population. This study aims to describe patterns and risks of medication use among pregnant women in mainland China with reference to the U.S. Food and Drug Administration (FDA) pregnancy risk category.
Methods: Hospital diagnostic and drug dispensing information of a national representative sample of basic medical insurance (BMI) beneficiaries was obtained from the China Health Insurance Association (CHIRA) database in 2015. Prevalence of use and number of medicines involved in each risk category were calculated. Most commonly used medicines from each risk category were illustrated. Factors associated with the use of category D/X medicines were evaluated through multiple logistic regression.
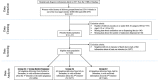
Results: Out of 11,373 women who had singleton deliveries in 2015, there were 2896 women with records covering their entire pregnancies, 5377, and 7946 women with records through the 2nd, and the 3rd trimester, respectively. It was found that 11.1% pregnant women used at least one medication and a total of 321 medications had been used during pregnancy. Most pregnant women used medicines which were classified FDA category C (66.2%), followed by category B (57.8%), category A (16.8%), category X (7.5%) and category D (5.0%). The most commonly used medicines from category D and X were anxiolytics and hormonal preparations respectively. Women who were from mid-western area (p = 0.045) or used four or more medications (p < 0.001) were more likely to use category D/X medicines.
Conclusions: This study revealed that about one in ten pregnant women used at least one medication during pregnancy in China and a significant number of them used FDA Category D or X medicines. The usage patterns identified in the present study indicate that sub-optimal medicine use might exist warranting further evaluation and intervention in future studies. More efforts are needed to uncover the safety concerns about medication use during pregnancy and improve current information system for clinical practice.
Keywords: China; FDA pregnancy risk category; Medication use; Pregnancy; Safety.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Ritchie H, Bolton P. The Australian categorisation of risk of drug use in pregnancy. Aust Fam Physician. 2000;29(3):237–241. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical