Bortezomib inhibits growth and sensitizes glioma to temozolomide (TMZ) via down-regulating the FOXM1-Survivin axis
- PMID: 31796105
- PMCID: PMC6892143
- DOI: 10.1186/s40880-019-0424-2
Bortezomib inhibits growth and sensitizes glioma to temozolomide (TMZ) via down-regulating the FOXM1-Survivin axis
Abstract
Background: High-grade glioma (HGG) is a fatal human cancer. Bortezomib, a proteasome inhibitor, has been approved for the treatment of multiple myeloma but its use in glioma awaits further investigation. This study aimed to explore the chemotherapeutic effect and the underlying mechanism of bortezomib on gliomas.
Methods: U251 and U87 cell viability and proliferation were detected by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay, tumor cell spheroid growth, and colony formation assay. Cell apoptosis and cell cycle were detected by flow cytometry. Temozolomide (TMZ)-insensitive cell lines were induced by long-term TMZ treatment, and cells with stem cell characteristics were enriched with stem cell culture medium. The mRNA levels of interested genes were measured via reverse transcription-quantitative polymerase chain reaction, and protein levels were determined via Western blotting/immunofluorescent staining in cell lines and immunohistochemical staining in paraffin-embedded sections. Via inoculating U87 cells subcutaneously, glioma xenograft models in nude mice were established for drug experiments. Patient survival data were analyzed using the Kaplan-Meier method.
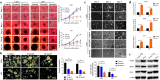
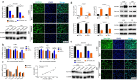
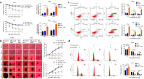
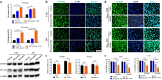
Results: Bortezomib inhibited the viability and proliferation of U251 and U87 cells in a dose- and time-dependent manner by inducing apoptosis and cell cycle arrest. Bortezomib also significantly inhibited the spheroid growth, colony formation, and stem-like cell proliferation of U251 and U87 cells. When administrated in combination, bortezomib showed synergistic effect with TMZ in vitro and sensitized glioma to TMZ treatment both in vitro and in vivo. Bortezomib reduced both the mRNA and protein levels of Forkhead Box M1 (FOXM1) and its target gene Survivin. The FOXM1-Survivin axis was markedly up-regulated in established TMZ-insensitive glioma cell lines and HGG patients. Expression levels of FOXM1 and Survivin were positively correlated with each other and both related to poor prognosis in glioma patients.
Conclusions: Bortezomib was found to inhibit glioma growth and improved TMZ chemotherapy efficacy, probably via down-regulating the FOXM1-Survivin axis. Bortezomib might be a promising agent for treating malignant glioma, alone or in combination with TMZ.
Keywords: Bortezomib; Chemotherapy; FOXM1; Glioma; Survivin; Temozolomide (TMZ).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
FOXM1-mediated NUF2 expression confers temozolomide resistance to human glioma cells by regulating autophagy via the PI3K/AKT/mTOR signaling pathway.Neuropathology. 2022 Oct;42(5):430-446. doi: 10.1111/neup.12824. Epub 2022 Jun 14. Neuropathology. 2022. PMID: 35701983
-
Resveratrol restores sensitivity of glioma cells to temozolamide through inhibiting the activation of Wnt signaling pathway.J Cell Physiol. 2019 May;234(5):6783-6800. doi: 10.1002/jcp.27409. Epub 2018 Oct 14. J Cell Physiol. 2019. PMID: 30317578
-
FoxM1-mediated RFC5 expression promotes temozolomide resistance.Cell Biol Toxicol. 2017 Dec;33(6):527-537. doi: 10.1007/s10565-017-9381-1. Epub 2017 Feb 9. Cell Biol Toxicol. 2017. Retraction in: Cell Biol Toxicol. 2024 Jun 8;40(1):43. doi: 10.1007/s10565-024-09887-0. PMID: 28185110 Retracted.
-
From Laboratory Studies to Clinical Trials: Temozolomide Use in IDH-Mutant Gliomas.Cells. 2021 May 17;10(5):1225. doi: 10.3390/cells10051225. Cells. 2021. PMID: 34067729 Free PMC article. Review.
-
Temozolomide sensitivity of malignant glioma cell lines - a systematic review assessing consistencies between in vitro studies.BMC Cancer. 2021 Nov 18;21(1):1240. doi: 10.1186/s12885-021-08972-5. BMC Cancer. 2021. PMID: 34794398 Free PMC article.
Cited by
-
Stemness subtypes in lower-grade glioma with prognostic biomarkers, tumor microenvironment, and treatment response.Sci Rep. 2024 Jun 26;14(1):14758. doi: 10.1038/s41598-024-65717-7. Sci Rep. 2024. PMID: 38926605 Free PMC article.
-
FAM84B promotes the proliferation of glioma cells through the cell cycle pathways.World J Surg Oncol. 2022 Nov 23;20(1):368. doi: 10.1186/s12957-022-02831-8. World J Surg Oncol. 2022. PMID: 36419094 Free PMC article.
-
Therapeutic Options in Neuro-Oncology.Int J Mol Sci. 2022 May 11;23(10):5351. doi: 10.3390/ijms23105351. Int J Mol Sci. 2022. PMID: 35628161 Free PMC article. Review.
-
Anticancer Activity of Amb4269951, a Choline Transporter-Like Protein 1 Inhibitor, in Human Glioma Cells.Pharmaceuticals (Basel). 2020 May 25;13(5):104. doi: 10.3390/ph13050104. Pharmaceuticals (Basel). 2020. PMID: 32466342 Free PMC article.
-
PDGF-D Promotes Epithelial-Mesenchymal Transition of Glioma Cells Through the NF-κB/NOTCH1 Pathway.Cancer Med. 2025 Jun;14(12):e71002. doi: 10.1002/cam4.71002. Cancer Med. 2025. PMID: 40530904 Free PMC article.
References
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJB, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

