Why Is Amyloid-β PET Requested After Performing CSF Biomarkers?
- PMID: 31796674
- PMCID: PMC7081099
- DOI: 10.3233/JAD-190836
Why Is Amyloid-β PET Requested After Performing CSF Biomarkers?
Abstract
Background: Amyloid-β positron emission tomography (PET) and cerebrospinal fluid (CSF) Aβ42 are considered interchangeable for clinical diagnosis of Alzheimer's disease.
Objective: To explore the clinical reasoning for requesting additional amyloid-β PET after performing CSF biomarkers.
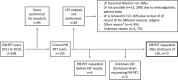
Methods: We retrospectively identified 72 memory clinic patients who underwent amyloid-β PET after CSF biomarkers analysis for clinical diagnostic evaluation between 2011 and 2019. We performed patient chart reviews to identify factors which led to additional amyloid-β PET. Additionally, we assessed accordance with appropriate-use-criteria (AUC) for amyloid-β PET.
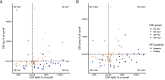
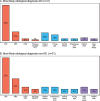
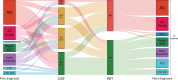
Results: Mean patient age was 62.0 (SD = 8.1) and mean Mini-Mental State Exam score was 23.6 (SD = 3.8). CSF analysis conflicting with the clinical diagnosis was the most frequent reason for requesting an amyloid-β PET scan (n = 53, 74%), followed by incongruent MRI (n = 16, 22%), unusual clinical presentation (n = 11, 15%) and young age (n = 8, 11%). An amyloid-β PET scan was rarely (n = 5, 7%) requested in patients with a CSF Aβ+/tau+ status. Fifteen (47%) patients with a post-PET diagnosis of AD had a predominantly non-amnestic presentation. In n = 11 (15%) cases, the reason that the clinician requested amyloid-β was not covered by AUC. This happened most often (n = 7) when previous CSF analysis did not support current clinical diagnosis, which led to requesting amyloid-β PET.
Conclusion: In this single-center study, the main reason for requesting an amyloid-β PET scan after performing CSF biomarkers was the occurrence of a mismatch between the primary clinical diagnosis and CSF Aβ/tau results.
Keywords: Alzheimer’s disease; amyloid; cerebrospinal fluid; positron emission tomography; tau proteins.
Conflict of interest statement
Authors’ disclosures available online (
Figures
Similar articles
-
Challenges in Alzheimer's Disease Diagnostic Work-Up: Amyloid Biomarker Incongruences.J Alzheimers Dis. 2020;77(1):203-217. doi: 10.3233/JAD-200119. J Alzheimers Dis. 2020. PMID: 32716357
-
Testing the 2018 NIA-AA research framework in a retrospective large cohort of patients with cognitive impairment: from biological biomarkers to clinical syndromes.Alzheimers Res Ther. 2019 Oct 15;11(1):84. doi: 10.1186/s13195-019-0543-7. Alzheimers Res Ther. 2019. PMID: 31615545 Free PMC article.
-
Reciprocal Predictive Relationships between Amyloid and Tau Biomarkers in Alzheimer's Disease Progression: An Empirical Model.J Neurosci. 2019 Sep 11;39(37):7428-7437. doi: 10.1523/JNEUROSCI.1056-19.2019. Epub 2019 Jul 26. J Neurosci. 2019. PMID: 31350262 Free PMC article.
-
Biomarkers in the Diagnosis and Prognosis of Alzheimer's Disease.J Lab Autom. 2015 Oct;20(5):589-600. doi: 10.1177/2211068214559979. Epub 2014 Nov 25. J Lab Autom. 2015. PMID: 25424384 Review.
-
Concordance between brain 18F-FDG PET and cerebrospinal fluid biomarkers in diagnosing Alzheimer's disease.Rev Esp Med Nucl Imagen Mol (Engl Ed). 2018 Jan-Feb;37(1):3-8. doi: 10.1016/j.remn.2017.05.003. Epub 2017 Jun 20. Rev Esp Med Nucl Imagen Mol (Engl Ed). 2018. PMID: 28645685 Review. English, Spanish.
Cited by
-
Clinical application of CSF biomarkers for Alzheimer's disease: From rationale to ratios.Alzheimers Dement (Amst). 2022 Apr 27;14(1):e12314. doi: 10.1002/dad2.12314. eCollection 2022. Alzheimers Dement (Amst). 2022. PMID: 35496374 Free PMC article.
-
Lecanemab approval in EU: what should we be ready for?- the EANM perspective.Eur J Nucl Med Mol Imaging. 2025 Apr;52(5):1607-1610. doi: 10.1007/s00259-025-07066-9. Eur J Nucl Med Mol Imaging. 2025. PMID: 39789225 Free PMC article. No abstract available.
-
Experiences from Clinical Research and Routine Use of Florbetaben Amyloid PET-A Decade of Post-Authorization Insights.Pharmaceuticals (Basel). 2024 Dec 7;17(12):1648. doi: 10.3390/ph17121648. Pharmaceuticals (Basel). 2024. PMID: 39770490 Free PMC article. Review.
-
Biomarkers Unveiling the Interplay of Mind, Nervous System, and Immunity.Methods Mol Biol. 2025;2868:73-90. doi: 10.1007/978-1-0716-4200-9_5. Methods Mol Biol. 2025. PMID: 39546226 Review.
-
A reproducible approach for the use of aptamer libraries for the identification of Aptamarkers for brain amyloid deposition based on plasma analysis.PLoS One. 2024 Aug 27;19(8):e0307678. doi: 10.1371/journal.pone.0307678. eCollection 2024. PLoS One. 2024. PMID: 39190656 Free PMC article.
References
-
- Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, Elliott C, Masliah E, Ryan L, Silverberg N (2018) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14, 535–562. - PMC - PubMed
-
- Ossenkoppele R, Jansen WJ, Rabinovici GD, Knol DL, van der Flier WM, van Berckel BNM, Scheltens P, Visser PJ, Verfaillie SCJ, Zwan MD, Adriaanse SM, Lammertsma AA, Barkhof F, Jagust WJ, Miller BL, Rosen HJ, Landau SM, Villemagne VL, Rowe CC, Lee DY, Na DL, Seo SW, Sarazin M, Roe CM, Sabri O, Barthel H, Koglin N, Hodges J, Leyton CE, Vandenberghe R, van Laere K, Drzezga A, Forster S, Grimmer T, Sánchez-Juan P, Carril JM, Mok V, Camus V, Klunk WE, Cohen AD, Meyer PT, Hellwig S, Newberg A, Frederiksen KS, Fleisher AS, Mintun MA, Wolk DA, Nordberg A, Rinne JO, Chételat G, Lleo A, Blesa R, Fortea J, Madsen K, Rodrigue KM, Brooks DJ (2015) Prevalence of amyloid PET positivity in dementia syndromes: A meta-analysis. JAMA 313, 1939–1949. - PMC - PubMed
-
- Strozyk D, Blennow K, White LR, Launer LJ (2003) CSF Abeta 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology 60, 652–656. - PubMed
-
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergström M, Savitcheva I, Huang GF, Estrada S, Ausén B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Långström B (2004) Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol 55, 306–319. - PubMed
-
- Blennow K, Mattsson N, Schöll M, Hansson O, Zetterberg H (2015) Amyloid biomarkers in Alzheimer’s disease. Trends Pharmacol Sci 36, 297–309. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

