Subendothelial stiffness alters endothelial cell traction force generation while exerting a minimal effect on the transcriptome
- PMID: 31796790
- PMCID: PMC6890669
- DOI: 10.1038/s41598-019-54336-2
Subendothelial stiffness alters endothelial cell traction force generation while exerting a minimal effect on the transcriptome
Abstract
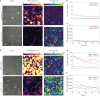
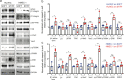
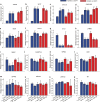
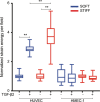
Endothelial cells respond to changes in subendothelial stiffness by altering their migration and mechanics, but whether those responses are due to transcriptional reprogramming remains largely unknown. We measured traction force generation and also performed gene expression profiling for two endothelial cell types grown in monolayers on soft or stiff matrices: primary human umbilical vein endothelial cells (HUVEC) and immortalized human microvascular endothelial cells (HMEC-1). Both cell types respond to changes in subendothelial stiffness by increasing the traction stresses they exert on stiffer as compared to softer matrices, and exhibit a range of altered protein phosphorylation or protein conformational changes previously implicated in mechanotransduction. However, the transcriptome has only a minimal role in this conserved biomechanical response. Only few genes were differentially expressed in each cell type in a stiffness-dependent manner, and none were shared between them. In contrast, thousands of genes were differentially regulated in HUVEC as compared to HMEC-1. HUVEC (but not HMEC-1) upregulate expression of TGF-β2 on stiffer matrices, and also respond to application of exogenous TGF-β2 by enhancing their endogenous TGF-β2 expression and their cell-matrix traction stresses. Altogether, these findings provide insights into the relationship between subendothelial stiffness, endothelial mechanics and variation of the endothelial cell transcriptome, and reveal that subendothelial stiffness, while critically altering endothelial cells' mechanical behavior, minimally affects their transcriptome.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Matrix stiffness exerts biphasic control over monocyte-endothelial adhesion via Rho-mediated ICAM-1 clustering.Integr Biol (Camb). 2016 Aug 8;8(8):869-78. doi: 10.1039/c6ib00084c. Epub 2016 Jul 22. Integr Biol (Camb). 2016. PMID: 27444067
-
Retinal pigment epithelium-derived transforming growth factor-β2 inhibits the angiogenic response of endothelial cells by decreasing vascular endothelial growth factor receptor-2 expression.J Cell Physiol. 2019 Apr;234(4):3837-3849. doi: 10.1002/jcp.27156. Epub 2018 Sep 7. J Cell Physiol. 2019. PMID: 30256387
-
The glycocalyx core protein Glypican 1 protects vessel wall endothelial cells from stiffness-mediated dysfunction and disease.Cardiovasc Res. 2021 May 25;117(6):1592-1605. doi: 10.1093/cvr/cvaa201. Cardiovasc Res. 2021. PMID: 32647868 Free PMC article.
-
The fate of renal allografts hinges on responses of the microvascular endothelium.Exp Mol Pathol. 2013 Apr;94(2):398-411. doi: 10.1016/j.yexmp.2012.06.002. Epub 2012 Jun 15. Exp Mol Pathol. 2013. PMID: 22710034
-
Exogenous and endogenous force regulation of endothelial cell behavior.J Biomech. 2010 Jan 5;43(1):79-86. doi: 10.1016/j.jbiomech.2009.09.012. Epub 2009 Oct 7. J Biomech. 2010. PMID: 19815215 Review.
Cited by
-
Endothelial Cells Potentially Participate in the Metastasis of Triple-Negative Breast Cancer.J Immunol Res. 2022 Feb 27;2022:5412007. doi: 10.1155/2022/5412007. eCollection 2022. J Immunol Res. 2022. PMID: 35265720 Free PMC article.
-
Substrate stiffness modulates migration and local intercellular membrane motion in pulmonary endothelial cell monolayers.Am J Physiol Cell Physiol. 2022 Sep 1;323(3):C936-C949. doi: 10.1152/ajpcell.00339.2021. Epub 2022 Aug 1. Am J Physiol Cell Physiol. 2022. PMID: 35912996 Free PMC article.
-
The Edifice of Vasculature-On-Chips: A Focused Review on the Key Elements and Assembly of Angiogenesis Models.ACS Biomater Sci Eng. 2024 Jun 10;10(6):3548-3567. doi: 10.1021/acsbiomaterials.3c01978. Epub 2024 May 7. ACS Biomater Sci Eng. 2024. PMID: 38712543 Free PMC article. Review.
-
Integration of Extracellular Matrices into Organ-on-Chip Systems.Adv Healthc Mater. 2023 Aug;12(20):e2203256. doi: 10.1002/adhm.202203256. Epub 2023 Apr 29. Adv Healthc Mater. 2023. PMID: 37018430 Free PMC article. Review.
-
Strategies for in situ tissue engineering of vascularized bone regeneration (Review).Biomed Rep. 2023 May 22;18(6):42. doi: 10.3892/br.2023.1625. eCollection 2023 Jun. Biomed Rep. 2023. PMID: 37325184 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

