Agastache honey has superior antifungal activity in comparison with important commercial honeys
- PMID: 31796803
- PMCID: PMC6890684
- DOI: 10.1038/s41598-019-54679-w
Agastache honey has superior antifungal activity in comparison with important commercial honeys
Abstract
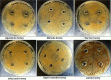
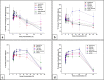
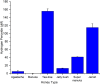
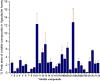
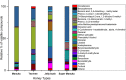
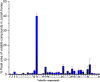
There is an urgent need for new effective antifungal agents suitable for the treatment of superficial skin infections, since acquired resistance of fungi to currently available agents is increasing. The antifungal activity of mono-floral Agastache honey and commercially available honeys were tested against dermatophytes (T. mentagrophytes and T. rubrum) and C. albicans (ATCC 10231 and a clinical isolate) by agar well diffusion and micro-dilution (AWD and MD). In AWD and MD assays, Agastache honey was effective at 40% concentration against dermatophytes (zone diameter, 19.5-20 mm) and C. albicans with the same MIC and MFC values indicating fungicidal activity. Tea tree honey was effective at 80% concentration (zone diameter, 14 mm) against dermatophytes and at 40% concentration against T. mentagrophytes and C. albicans. Manuka was effective at 80% concentration only against T. mentagrophytes (zone diameter, 12 mm) and at 40% against T. rubrum and C. albicans with fungistatic activity. Similar to the AWD results, Jelly bush, Super Manuka, and Jarrah showed no activity against dermatophytes but showed some activity against C. albicans. Headspace volatiles of six honeys were isolated by SPME and identified by GC-MS. The characteristic chemical markers for each honey were as follows: Agastache- Phenol, 2,4-bis(1,1-dimethylethyl) and Estragole; Manuka and Tea-tree- Acetanisole and Methyl 3,5-dimethoxybenzoate; Jelly bush- Linalool and Nonanal; Super Manuka- Methyl 3,5-dimethoxybenzoate and Nonanal; Jarrah- Isophorone and Nonanoic acid. Overall, analysis of the bioactive compound content and antifungal activity of Agastache honey indicated possible use as an antifungal agent for management of superficial fungal infections.
Conflict of interest statement
Author George Livanos was employed by company Kenkay Pharmaceuticals. All other authors declare no competing interests. Kenkay Pharmaceuticals partially funded this work through Australian Research Council’s Linkage Project scheme (Grant number LP120200743).
Figures

References
-
- Chakraborti, T., Barman, S. & Mandal, N. C. Original Research Article Evaluation of antibacterial potential of some Indian honey samples against throat and skin infective pathogens. 3, 362–369 (2014).
-
- Sell Scott A., Wolfe Patricia S., Spence Andrew J., Rodriguez Isaac A., McCool Jennifer M., Petrella Rebecca L., Garg Koyal, Ericksen Jeffery J., Bowlin Gary L. A Preliminary Study on the Potential of Manuka Honey and Platelet-Rich Plasma in Wound Healing. International Journal of Biomaterials. 2012;2012:1–14. doi: 10.1155/2012/313781. - DOI - PMC - PubMed
-
- Lund-Nielsen Betina, Adamsen Lis, Kolmos Hans Jørn, Rørth Mikael, Tolver Anders, Gottrup Finn. The effect of honey-coated bandages compared with silver-coated bandages on treatment of malignant wounds-a randomized study. Wound Repair and Regeneration. 2011;19(6):664–670. doi: 10.1111/j.1524-475X.2011.00735.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

