FOXO3a regulates rhinovirus-induced innate immune responses in airway epithelial cells
- PMID: 31796819
- PMCID: PMC6890790
- DOI: 10.1038/s41598-019-54567-3
FOXO3a regulates rhinovirus-induced innate immune responses in airway epithelial cells
Abstract
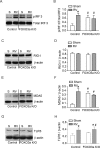
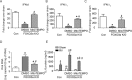
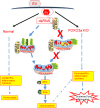
Forkhead transcription factor class O (FOXO)3a, which plays a critical role in a wide variety of cellular processes, was also found to regulate cell-type-specific antiviral responses. Airway epithelial cells express FOXO3a and play an important role in clearing rhinovirus (RV) by mounting antiviral type I and type III interferon (IFN) responses. To elucidate the role of FOXO3a in regulating antiviral responses, we generated airway epithelial cell-specific Foxo3a knockout (Scga1b1-Foxo3a-/-) mice and a stable FOXO3a knockout human airway epithelial cell line. Compared to wild-type, Scga1b1-Foxo3a-/- mice show reduced IFN-α, IFN-β, IFN-λ2/3 in response to challenge with RV or double-stranded (ds)RNA mimic, Poly Inosinic-polycytidylic acid (Poly I:C) indicating defective dsRNA receptor signaling. RV-infected Scga1b1-Foxo3a-/- mice also show viral persistence, enhanced lung inflammation and elevated pro-inflammatory cytokine levels. FOXO3a K/O airway epithelial cells show attenuated IFN responses to RV infection and this was associated with conformational change in mitochondrial antiviral signaling protein (MAVS) but not with a reduction in the expression of dsRNA receptors under unstimulated conditions. Pretreatment with MitoTEMPO, a mitochondrial-specific antioxidant corrects MAVS conformation and restores antiviral IFN responses to subsequent RV infection in FOXO3a K/O cells. Inhibition of oxidative stress also reduces pro-inflammatory cytokine responses to RV in FOXO3a K/O cells. Together, our results indicate that FOXO3a plays a critical role in regulating antiviral responses as well as limiting pro-inflammatory cytokine expression. Based on these results, we conclude that FOXO3a contributes to optimal viral clearance and prevents excessive lung inflammation following RV infection.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

