The therapeutic efficacy of Bifidobacterium animalis subsp. lactis BB-12® in infant colic: A randomised, double blind, placebo-controlled trial
- PMID: 31797399
- PMCID: PMC6973258
- DOI: 10.1111/apt.15561
The therapeutic efficacy of Bifidobacterium animalis subsp. lactis BB-12® in infant colic: A randomised, double blind, placebo-controlled trial
Abstract
Background: The pathogenesis of infant colic is poorly defined. Gut microbiota seems to be involved, supporting the potential therapeutic role of probiotics.
Aims: To assess the rate of infants with a reduction of ≥50% of mean daily crying duration after 28 days of intervention with the probiotic Bifidobacterium animalis subsp. lactis BB-12® (BB-12). Secondary outcomes were daily number of crying episodes, sleeping time, number of bowel movements and stool consistency.
Methods: Randomized controlled trial (RCT) on otherwise healthy exclusively breastfed infants with infant colic randomly allocated to receive BB-12 (1 × 109 CFU/day) or placebo for 28 days. Gut microbiota structure and butyrate, beta-defensin-2 (HBD-2), cathelicidin (LL-37), secretory IgA (sIgA) and faecal calprotectin levels were assessed.
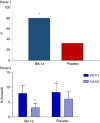
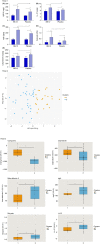
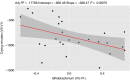
Results: Eighty infants were randomised, 40/group. The rate of infants with reduction of ≥50% of mean daily crying duration was higher in infants treated with BB-12, starting from the end of 2nd week. No infant relapsed when treatment was stopped. The mean number of crying episodes decreased in both groups, but with a higher effect in BB-12 group (-4.7 ± 3.4 vs -2.3 ± 2.2, P < 0.05). Mean daily stool frequency decreased in both groups but the effect was significantly higher in the BB-12 group; stool consistency was similar between the two groups. An increase in Bifidobacterium abundance (with significant correlation with crying time reduction), butyrate and HBD-2, LL-37, sIgA levels associated with a decrease in faecal calprotectin level were observed in the BB-12 group.
Conclusions: Supplementation with BB-12 is effective in managing infant colic. The effect could derive from immune and non-immune mechanisms associated with a modulation of gut microbiota structure and function.
© 2019 The Authors. Alimentary Pharmacology & Therapeutics Published by John Wiley & Sons Ltd.
Figures
Comment in
-
Editorial: interventions in infantile colic - can efficacy be attributed to treatment or to time? Authors' reply.Aliment Pharmacol Ther. 2020 Feb;51(3):398-399. doi: 10.1111/apt.15627. Aliment Pharmacol Ther. 2020. PMID: 31943267 No abstract available.
-
Editorial: interventions in infantile colic - can efficacy be attributed to treatment or to time?Aliment Pharmacol Ther. 2020 Feb;51(3):397-398. doi: 10.1111/apt.15599. Aliment Pharmacol Ther. 2020. PMID: 31943271 No abstract available.
References
-
- Wolke D, Bilgin A, Samara M. Systematic review and meta‐analysis: fussing and crying durations and prevalence of colic in infants. J Pediatr. 2017;185:e4. - PubMed
-
- James‐Roberts IS. In Barr RG, St James‐Roberts I, Keefe MR, eds. New Evidence on Unexplained Early Infant Crying: Its Origins, Nature and Management. Skillman, New Jersey: Johnson & Johnson Pediatric Institute; 2001:5‐24.
-
- Johnson JD, Cocker K, Chang E. Infantile colic: recognition and treatment. Am Fam Physician. 2015;92:577‐582. - PubMed
-
- Zeevenhooven J, Browne PD, L'Hoir MP, de Weerth C, Benninga MA. Infant colic: mechanisms and management. Nat Rev Gastroenterol Hepatol. 2018;15:479‐496. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
