Efficacy and safety of insulin glargine 300 U/mL versus insulin glargine 100 U/mL in Asia Pacific insulin-naïve people with type 2 diabetes: The EDITION AP randomized controlled trial
- PMID: 31797549
- PMCID: PMC7384042
- DOI: 10.1111/dom.13936
Efficacy and safety of insulin glargine 300 U/mL versus insulin glargine 100 U/mL in Asia Pacific insulin-naïve people with type 2 diabetes: The EDITION AP randomized controlled trial
Abstract
Aim: To compare the efficacy and safety of Gla-300 versus Gla-100 in insulin-naïve people with type 2 diabetes in Asia Pacific.
Materials and methods: In this open-label, randomized, active-controlled, 26-week study, insulin-naïve participants with type 2 diabetes inadequately controlled with non-insulin antihyperglycaemic drugs were randomized (2:1) to Gla-300 or Gla-100. The initial daily dose of basal insulin was 0.2 U/kg and was adjusted at least weekly for 8-12 weeks to a target fasting self-monitored plasma glucose (SMPG) of 4.4-5.6 mmol/L.
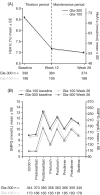
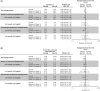
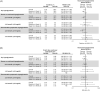
Results: Of the 604 participants randomized, 570 (Gla-300, n = 375; Gla-100, n = 195) completed the study. Non-inferiority of Gla-300 versus Gla-100 in HbA1c reduction from baseline to week 26 was confirmed. In the Gla-300 and Gla-100 groups, 51.1% and 52.2% of participants achieved the HbA1c target of <7.0% (rate ratio [95% CI]: 0.98 [0.84 to 1.14]) and 19.1% and 21.9% achieved the target without hypoglycaemia during the last 12 weeks of treatment (rate ratio [95% CI]: 0.87 [0.63 to 1.20]). Changes in fasting plasma glucose and 24-hour average eight-point SMPG were comparable between groups. Incidence of hypoglycaemia at any time of day was similar between treatment groups at week 26, but incidence of any nocturnal hypoglycaemia was numerically lower with Gla-300 than Gla-100 over the initial 12-week titration period and 26-week on-treatment period. Rates of adverse events were similar between groups and low for serious adverse events.
Conclusions: Glycaemic control of Gla-300 is non-inferior to Gla-100 with a similar or lower incidence and proportion of hypoglycaemia in people with type 2 diabetes in Asia Pacific, reinforcing the results in the global EDITION programme.
Keywords: basal insulin; glycaemic control; hypoglycaemia; insulin analogues; insulin therapy; population study.
© 2019 John Wiley & Sons Ltd.
Conflict of interest statement
L.J. has received honoraria and consultancy fees from Sanofi. S.S. is an employee of Sanofi. E.N. is an employee and shareholder of Sanofi. E.S.K., X.D., L.L. and G.Y. have nothing to declare.
Figures
References
-
- International Diabetes Federation (IDF) . IDF Diabetes Atlas 8th ed. 2017. http://diabetesatlas.org/. Accessed March 13, 2019.
-
- Jia W, Weng J, Zhu D, et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab Res Rev. 2019;e3158:35. - PubMed
-
- Chiang C‐E, Lin S‐Y, Lin T‐H, et al. 2018 consensus of the Taiwan Society of Cardiology and the Diabetes Association of Republic of China (Taiwan) on the pharmacological management of patients with type 2 diabetes and cardiovascular diseases. J Chinese Med Assoc. 2018;81:189‐222. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

