Whole-genome comparison between the type strain of Halobacterium salinarum (DSM 3754T ) and the laboratory strains R1 and NRC-1
- PMID: 31797576
- PMCID: PMC7002104
- DOI: 10.1002/mbo3.974
Whole-genome comparison between the type strain of Halobacterium salinarum (DSM 3754T ) and the laboratory strains R1 and NRC-1
Abstract
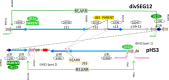
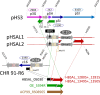
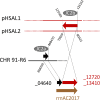
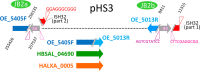
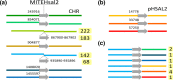
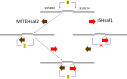
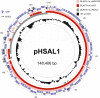
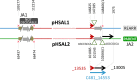
Halobacterium salinarum is an extremely halophilic archaeon that is widely distributed in hypersaline environments and was originally isolated as a spoilage organism of salted fish and hides. The type strain 91-R6 (DSM 3754T ) has seldom been studied and its genome sequence has only recently been determined by our group. The exact relationship between the type strain and two widely used model strains, NRC-1 and R1, has not been described before. The genome of Hbt. salinarum strain 91-R6 consists of a chromosome (2.17 Mb) and two large plasmids (148 and 102 kb, with 39,230 bp being duplicated). Cytosine residues are methylated (m4 C) within CTAG motifs. The genomes of type and laboratory strains are closely related, their chromosomes sharing average nucleotide identity (ANIb) values of 98% and in silico DNA-DNA hybridization (DDH) values of 95%. The chromosomes are completely colinear, do not show genome rearrangement, and matching segments show <1% sequence difference. Among the strain-specific sequences are three large chromosomal replacement regions (>10 kb). The well-studied AT-rich island (61 kb) of the laboratory strains is replaced by a distinct AT-rich sequence (47 kb) in 91-R6. Another large replacement (91-R6: 78 kb, R1: 44 kb) codes for distinct homologs of proteins involved in motility and N-glycosylation. Most (107 kb) of plasmid pHSAL1 (91-R6) is very closely related to part of plasmid pHS3 (R1) and codes for essential genes (e.g. arginine-tRNA ligase and the pyrimidine biosynthesis enzyme aspartate carbamoyltransferase). Part of pHS3 (42.5 kb total) is closely related to the largest strain-specific sequence (164 kb) in the type strain chromosome. Genome sequencing unraveled the close relationship between the Hbt. salinarum type strain and two well-studied laboratory strains at the DNA and protein levels. Although an independent isolate, the type strain shows a remarkably low evolutionary difference to the laboratory strains.
Keywords: comparative genomics; genomic variability; haloarchaea; halobacteria; megaplasmid; type strain.
© 2019 The Authors. MicrobiologyOpen published by John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures





References
-
- Abdul Halim, M. F. , Pfeiffer, F. , Zou, J. , Frisch, A. , Haft, D. , Wu, S. , … Pohlschroder, M. (2013). Haloferax volcanii archaeosortase is required for motility, mating, and C‐terminal processing of the S‐layer glycoprotein. Molecular Microbiology, 88, 1164–1175. - PubMed
-
- Aivaliotis, M. , Gevaert, K. , Falb, M. , Tebbe, A. , Konstantinidis, K. , Bisle, B. , … Oesterhelt, D. (2007). Large‐scale identification of N‐terminal peptides in the halophilic archaea Halobacterium salinarum and Natronomonas pharaonis . Journal of Proteome Research, 6, 2195–2204. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

