Serum-circulating His-tRNA synthetase inhibits organ-targeted immune responses
- PMID: 31797905
- PMCID: PMC8166958
- DOI: 10.1038/s41423-019-0331-0
Serum-circulating His-tRNA synthetase inhibits organ-targeted immune responses
Abstract
His-tRNA synthetase (HARS) is targeted by autoantibodies in chronic and acute inflammatory anti-Jo-1-positive antisynthetase syndrome. The extensive activation and migration of immune cells into lung and muscle are associated with interstitial lung disease, myositis, and morbidity. It is unknown whether the sequestration of HARS is an epiphenomenon or plays a causal role in the disease. Here, we show that HARS circulates in healthy individuals, but it is largely undetectable in the serum of anti-Jo-1-positive antisynthetase syndrome patients. In cultured primary human skeletal muscle myoblasts (HSkMC), HARS is released in increasing amounts during their differentiation into myotubes. We further show that HARS regulates immune cell engagement and inhibits CD4+ and CD8+ T-cell activation. In mouse and rodent models of acute inflammatory diseases, HARS administration downregulates immune activation. In contrast, neutralization of extracellular HARS by high-titer antibody responses during tissue injury increases susceptibility to immune attack, similar to what is seen in humans with anti-Jo-1-positive disease. Collectively, these data suggest that extracellular HARS is homeostatic in normal subjects, and its sequestration contributes to the morbidity of the anti-Jo-1-positive antisynthetase syndrome.
Keywords: Autoimmunity; HARS; Immunology; Synthetase; tRNA.
Conflict of interest statement
The authors declare no competing interests.
Figures

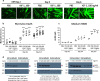
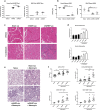
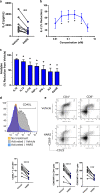
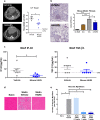
References
-
- Hervier B, Benveniste O. Clinical heterogeneity and outcomes of antisynthetase syndrome. Curr. Rheumatol. Rep. 2013;15:349. - PubMed
-
- Mathews MB, Bernstein RM. Myositis autoantibody inhibits histidyl-tRNA synthetase: a model for autoimmunity. Nature. 1983;304:177–179. - PubMed
-
- Ramsden DA, et al. Epitope mapping of the cloned human autoantigen, histidyl-tRNA synthetase. Analysis of the myositis-associated anti-Jo-1 autoimmune response. J. Immunol. 1989;143:2267–2272. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- R01 GM088278/GM/NIGMS NIH HHS/United States
- R01 GM125908/GM/NIGMS NIH HHS/United States
- 1R01GM125908-01A1/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- 5R01GM088278-08/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

