Photo-activated raster scanning thermal imaging at sub-diffraction resolution
- PMID: 31797931
- PMCID: PMC6892803
- DOI: 10.1038/s41467-019-13447-0
Photo-activated raster scanning thermal imaging at sub-diffraction resolution
Abstract
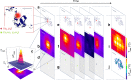
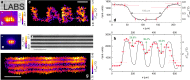
Active thermal imaging is a valuable tool for the nondestructive characterization of the morphological properties and the functional state of biological tissues and synthetic materials. However, state-of-the-art techniques do not typically combine the required high spatial resolution over extended fields of view with the quantification of temperature variations. Here, we demonstrate quantitative far-infrared photo-thermal imaging at sub-diffraction resolution over millimeter-sized fields of view. Our approach combines the sample absorption of modulated raster-scanned laser light with the automated localization of the laser-induced temperature variations imaged by a thermal camera. With temperature increments ∼0.5-5 °C, we achieve a six-time gain with respect to our 350-μm diffraction-limited resolution with proof-of-principle experiments on synthetic samples. We finally demonstrate the biological relevance of sub-diffraction thermal imaging by retrieving temperature-based super-resolution maps of the distribution of Prussian blue nanocubes across explanted murine skin biopsies.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Lahiri BB, et al. Quantification of defects in composites and rubber materials using active thermography. Infrared Phys. Technol. 2012;55:191–199. doi: 10.1016/j.infrared.2012.01.001. - DOI
Publication types
LinkOut - more resources
Full Text Sources

