Dynamic and tunable metabolite control for robust minimal-equipment assessment of serum zinc
- PMID: 31797936
- PMCID: PMC6892929
- DOI: 10.1038/s41467-019-13454-1
Dynamic and tunable metabolite control for robust minimal-equipment assessment of serum zinc
Abstract
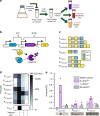
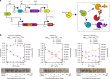
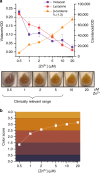
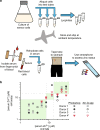
Bacterial biosensors can enable programmable, selective chemical production, but difficulties incorporating metabolic pathways into complex sensor circuits have limited their development and applications. Here we overcome these challenges and present the development of fast-responding, tunable sensor cells that produce different pigmented metabolites based on extracellular concentrations of zinc (a critical micronutrient). We create a library of dual-input synthetic promoters that decouple cell growth from zinc-specific metabolite production, enabling visible cell coloration within 4 h. Using additional transcriptional and metabolic control methods, we shift the response thresholds by an order of magnitude to measure clinically relevant zinc concentrations. The resulting sensor cells report zinc concentrations in individual donor serum samples; we demonstrate that they can provide results in a minimal-equipment fashion, serving as the basis for a field-deployable assay for zinc deficiency. The presented advances are likely generalizable to the creation of other types of sensors and diagnostics.
Conflict of interest statement
M.P.S. is founder and officer of a company, Chromanostics, previously formed for commercialization of micronutrient diagnostics. The authors declare no other competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

