Characterising an Alternative Murine Model of Diabetic Cardiomyopathy
- PMID: 31798462
- PMCID: PMC6868003
- DOI: 10.3389/fphys.2019.01395
Characterising an Alternative Murine Model of Diabetic Cardiomyopathy
Erratum in
-
Corrigendum: Characterising an Alternative Murine Model of Diabetic Cardiomyopathy.Front Physiol. 2021 Aug 19;12:734320. doi: 10.3389/fphys.2021.734320. eCollection 2021. Front Physiol. 2021. PMID: 34489742 Free PMC article.
Abstract
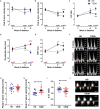
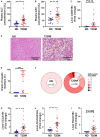
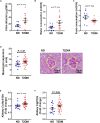
The increasing burden of heart failure globally can be partly attributed to the increased prevalence of diabetes, and the subsequent development of a distinct form of heart failure known as diabetic cardiomyopathy. Despite this, effective treatment options have remained elusive, due partly to the lack of an experimental model that adequately mimics human disease. In the current study, we combined three consecutive daily injections of low-dose streptozotocin with high-fat diet, in order to recapitulate the long-term complications of diabetes, with a specific focus on the diabetic heart. At 26 weeks of diabetes, several metabolic changes were observed including elevated blood glucose, glycated haemoglobin, plasma insulin and plasma C-peptide. Further analysis of organs commonly affected by diabetes revealed diabetic nephropathy, underlined by renal functional and structural abnormalities, as well as progressive liver damage. In addition, this protocol led to robust left ventricular diastolic dysfunction at 26 weeks with preserved systolic function, a key characteristic of patients with type 2 diabetes-induced cardiomyopathy. These observations corresponded with cardiac structural changes, namely an increase in myocardial fibrosis, as well as activation of several cardiac signalling pathways previously implicated in disease progression. It is hoped that development of an appropriate model will help to understand some the pathophysiological mechanisms underlying the accelerated progression of diabetic complications, leading ultimately to more efficacious treatment options.
Keywords: cardiac; diabetes; diabetic cardiomyopathy; experimental model; type 2 diabetes.
Copyright © 2019 Tate, Prakoso, Willis, Peng, Deo, Qin, Walsh, Nash, Cohen, Rofe, Sharma, Kiriazis, Donner, De Haan, Watson, De Blasio and Ritchie.
Figures



References
-
- De Blasio M.J., Ramalingam A., Cao A. H., Prakoso D., Ye J.-M., Pickering R., et al. (2017). The superoxide dismutase mimetic tempol blunts diabetes-induced upregulation of NADPH oxidase and endoplasmic reticulum stress in a rat model of diabetic nephropathy. Eur. J. Pharmacol. 807 12–20. 10.1016/j.ejphar.2017.04.026 - DOI - PubMed
LinkOut - more resources
Full Text Sources

