Neutrophil Extracellular Traps Induce Tissue-Invasive Monocytes in Granulomatosis With Polyangiitis
- PMID: 31798577
- PMCID: PMC6874157
- DOI: 10.3389/fimmu.2019.02617
Neutrophil Extracellular Traps Induce Tissue-Invasive Monocytes in Granulomatosis With Polyangiitis
Abstract
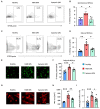
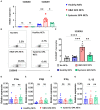
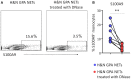
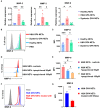
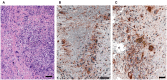
Objective: Granulomatosis with polyangiitis (GPA) is a multi-organ vasculitic syndrome typically associated with neutrophil extracellular trap (NET) formation and aggressive tissue inflammation. Manifestations in head and neck (H&N) GPA include septal perforations, saddle-nose deformities, bony erosions of the orbital and sinus walls, middle ear damage and epiglottitis, indicative of bone, cartilage, and connective tissue destruction. Whether H&N-centric lesions engage disease pathways distinctive from the ischemic tissue damage in the lungs, kidneys, skin, and peripheral nerves is unknown. We have compared inflammatory responses triggered by neutrophilic NETs in patients with H&N GPA and systemic GPA (sGPA). Methods: Neutrophils and monocytes were isolated from the peripheral blood of patients with H&N GPA, sGPA, and age/gender matched healthy individuals. Neutrophil NETosis was induced. NETs were isolated and cocultured with monocytes. Gene induction was quantified by RT-PCR, protein upregulation by flow cytometry. Tissue invasiveness of monocytes was measured in a 3D collagen matrix system. Expression of MMP-9 in tissue-residing macrophages was assessed by immunohistochemistry in tissue biopsies. Results: Neutrophils from H&N GPA patients showed more intense NETosis with higher frequencies of netting neutrophils (P < 0.001) and release of higher amounts of NETs (P < 0.001). Isolated NETs from H&N GPA functioned as an inducer of danger-associated molecular patterns in monocytes; specifically, alarmin S100A9. NET-induced upregulation of monocyte S100A9 required recognition of DNA. S100A9 release resulted in the induction of metalloproteinases, including MMP-9, and enabled monocytes to invade into extracellular matrix. Anti-MMP-9 treatment attenuated the tissue invasiveness of monocytes primed with NETs from H&N GPA patients. MMP-9-producing macrophages dominated the tissue infiltrates in naso-sinal biopsies from H&N GPA patients. Conclusion: Distinct disease patterns in GPA are associated with differences in NET formation and NET content. H&N GPA patients with midline cartilaginous and bony lesions are highly efficient in generating NETs. H&N GPA neutrophils trigger the induction of the alarmin S100A9, followed by production of MMP-9, endowing monocytes with tissue-invasive capabilities.
Keywords: NETosis; S100A9; bone destruction; cartilage destruction; granulomatosis with polyangiitis; matrix metalloproteinases; monocytes.
Copyright © 2019 Akiyama, Zeisbrich, Ibrahim, Ohtsuki, Berry, Hwang, Goronzy and Weyand.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

