Mechanisms of Relapse After CD19 CAR T-Cell Therapy for Acute Lymphoblastic Leukemia and Its Prevention and Treatment Strategies
- PMID: 31798590
- PMCID: PMC6863137
- DOI: 10.3389/fimmu.2019.02664
Mechanisms of Relapse After CD19 CAR T-Cell Therapy for Acute Lymphoblastic Leukemia and Its Prevention and Treatment Strategies
Abstract
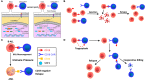
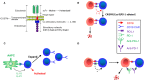
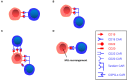
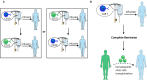
Chimeric antigen receptor (CAR) T-cell therapy is highly effective in the treatment of B-cell acute lymphoblastic leukemia (ALL) or B-cell lymphoma, providing alternative therapeutic options for patients who failed to respond to conventional treatment or relapse. Moreover, it can bridge other therapeutic strategies and greatly improve patient prognosis, with broad applicable prospects. Even so, 30-60% patients relapse after treatment, probably due to persistence of CAR T-cells and escape or downregulation of CD19 antigen, which is a great challenge for disease control. Therefore, understanding the mechanisms that underlie post-CAR relapse and establishing corresponding prevention and treatment strategies is important. Herein, we discuss post-CAR relapse from the aspects of CD19-positive and CD19-negative and provide some reasonable prevention and treatment strategies.
Keywords: CD19 CAR T-cell; mechanisms; prevention; relapse; strategies; treatment.
Copyright © 2019 Xu, Sun, Liang, Chen, Zhang, Zhou, Li, Tu, Liu, Tu and Li.
Figures
References
-
- Sotillo E, Barrett DM, Black KL, Bagashev A, Oldridge D, Wu G, et al. . Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. (2015) 5:1282–95. 10.1158/2159-8290.CD-15-1020 - DOI - PMC - PubMed
-
- Brentjens RJ, Rivière I, Park JH, Davila ML, Wang X, Stefanski J, et al. . Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. (2011) 118: 4817–28. 10.1182/blood-2011-04-348540 - DOI - PMC - PubMed
-
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. . T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. (2015) 385:517–28. 10.1016/S0140-6736(14)61403-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

