Poly(lactic-co-glycolic acid)-bioactive glass composites as nanoporous scaffolds for bone tissue engineering: In vitro and in vivo studies
- PMID: 31798710
- PMCID: PMC6880429
- DOI: 10.3892/etm.2019.8121
Poly(lactic-co-glycolic acid)-bioactive glass composites as nanoporous scaffolds for bone tissue engineering: In vitro and in vivo studies
Abstract
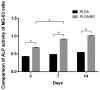
The aim of the present study was to investigate the feasibility of using composite scaffolds of poly(lactic-co-glycolic acid) (PLGA) and bioactive glass (BG) to repair bone defects. PLGA/BG composite scaffolds were prepared by thermally-induced phase separation. Scanning electron microscopy (SEM) was used to study the morphology, and liquid (absolute ethanol) replacement was used to calculate the porosity of the scaffold. The biocompatibility and degradation of the scaffold were determined using human osteosarcoma cell line MG-63 and animal experiments. SEM showed that the scaffold had a nanofibrous three-dimensional network structure with a fiber diameter of 160-320 nm, a pore size of 1-7 µm, and a porosity of 93.048±0.121%. The scaffold structure was conducive to cell adhesion and proliferation. It promoted cell osteogenesis and could be stably degraded in vivo.
Keywords: bioactive glass; bone tissue engineering; poly(lactic-co-glycolic acid); scaffold.
Copyright: © Yang et al.
Figures





References
LinkOut - more resources
Full Text Sources
