Imaging hair cells through laser-ablated cochlear bone
- PMID: 31799058
- PMCID: PMC6865115
- DOI: 10.1364/BOE.10.005974
Imaging hair cells through laser-ablated cochlear bone
Abstract
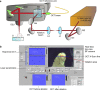
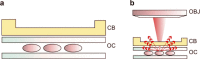
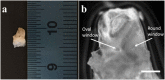
We report an innovative technique for the visualization of cells through an overlying scattering medium by combining femtosecond laser bone ablation and two-photon excitation fluorescence (TPEF) microscopy. We demonstrate the technique by imaging hair cells in an intact mouse cochlea ex vivo. Intracochlear imaging is important for the assessment of hearing disorders. However, the small size of the cochlea and its encasement in the densest bone in the body present challenging obstacles, preventing the visualization of the intracochlear microanatomy using standard clinical imaging modalities. The controlled laser ablation reduces the optical scattering of the cochlear bone while the TPEF allows visualization of individual cells behind the bone. We implemented optical coherence tomography (OCT) simultaneously with the laser ablation to enhance the precision of the ablation and prevent inadvertent damage to the cells behind the bone.
© 2019 Optical Society of America under the terms of the OSA Open Access Publishing Agreement.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Optical Coherence Tomography-Based Atlas of the Human Cochlear Hook Region.J Clin Med. 2022 Dec 28;12(1):238. doi: 10.3390/jcm12010238. J Clin Med. 2022. PMID: 36615042 Free PMC article.
-
Mammalian Cochlear Hair Cell Imaging Using Optical Coherence Tomography (OCT): A Preliminary Study.J Int Adv Otol. 2021 Jan;17(1):46-51. doi: 10.5152/iao.2020.8377. J Int Adv Otol. 2021. PMID: 33605221 Free PMC article.
-
Two-photon microscopy of the mouse cochlea in situ for cellular diagnosis.J Biomed Opt. 2013 Mar;18(3):31104. doi: 10.1117/1.JBO.18.3.031104. J Biomed Opt. 2013. PMID: 23165736
-
A chemo-mechanical cochleostomy preserves hearing for the in vivo functional imaging of cochlear cells.Nat Protoc. 2023 Apr;18(4):1137-1154. doi: 10.1038/s41596-022-00786-4. Epub 2023 Jan 4. Nat Protoc. 2023. PMID: 36599963 Review.
-
Theranostic applications of optical coherence tomography in neurosurgery?Neurosurg Rev. 2022 Feb;45(1):421-427. doi: 10.1007/s10143-021-01599-x. Epub 2021 Aug 16. Neurosurg Rev. 2022. PMID: 34398385 Free PMC article. Review.
Cited by
-
Two Photon Fluorescence Microscopy of the Unstained Human Cochlea Reveals Organ of Corti Cytoarchitecture.Front Cell Neurosci. 2021 Aug 5;15:690953. doi: 10.3389/fncel.2021.690953. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34421541 Free PMC article.
References
-
- Mosk A. P., Lagendijk A., Lerosey G., Fink M., “Controlling waves in space and time for imaging and focusing in complex media,” Nat. Photonics 6(5), 283–292 (2012).10.1038/nphoton.2012.88 - DOI
LinkOut - more resources
Full Text Sources
