Adult Stem Cells for Bone Regeneration and Repair
- PMID: 31799249
- PMCID: PMC6863062
- DOI: 10.3389/fcell.2019.00268
Adult Stem Cells for Bone Regeneration and Repair
Abstract
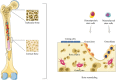
The regeneration of bone fractures, resulting from trauma, osteoporosis or tumors, is a major problem in our super-aging society. Bone regeneration is one of the main topics of concern in regenerative medicine. In recent years, stem cells have been employed in regenerative medicine with interesting results due to their self-renewal and differentiation capacity. Moreover, stem cells are able to secrete bioactive molecules and regulate the behavior of other cells in different host tissues. Bone regeneration process may improve effectively and rapidly when stem cells are used. To this purpose, stem cells are often employed with biomaterials/scaffolds and growth factors to accelerate bone healing at the fracture site. Briefly, this review will describe bone structure and the osteogenic differentiation of stem cells. In addition, the role of mesenchymal stem cells for bone repair/regrowth in the tissue engineering field and their recent progress in clinical applications will be discussed.
Keywords: bone; differentiation; regenerative medicine; repair; stem cell.
Copyright © 2019 Iaquinta, Mazzoni, Bononi, Rotondo, Mazziotta, Montesi, Sprio, Tampieri, Tognon and Martini.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

