Intestinal-level anti-inflammatory bioactivities of catechin-rich green tea: Rationale, design, and methods of a double-blind, randomized, placebo-controlled crossover trial in metabolic syndrome and healthy adults
- PMID: 31799477
- PMCID: PMC6881604
- DOI: 10.1016/j.conctc.2019.100495
Intestinal-level anti-inflammatory bioactivities of catechin-rich green tea: Rationale, design, and methods of a double-blind, randomized, placebo-controlled crossover trial in metabolic syndrome and healthy adults
Erratum in
-
Erratum regarding missing Declaration of Competing Interest statements in previously published articles.Contemp Clin Trials Commun. 2020 Dec 10;20:100689. doi: 10.1016/j.conctc.2020.100689. eCollection 2020 Dec. Contemp Clin Trials Commun. 2020. PMID: 33392413 Free PMC article.
Abstract
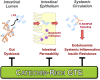
Metabolic endotoxemia initiates low-grade chronic inflammation in metabolic syndrome (MetS) and provokes the progression towards more advanced cardiometabolic disorders. Our recent works in obese rodent models demonstrate that catechin-rich green tea extract (GTE) improves gut barrier integrity to alleviate the translocation of gut-derived endotoxin and its consequent pro-inflammatory responses mediated through Toll-like receptor-4/nuclear factor κB (TLR4/NFκB) signaling. The objective of this clinical trial is to establish the efficacy of GTE to alleviate metabolic endotoxemia-associated inflammation in persons with MetS by improving gut barrier function. We plan a double-blind, placebo-controlled cross-over trial in persons with MetS and age- and gender-matched healthy persons (18-65 y; n = 20/group) who will receive a low-energy GTE-rich (1 g/day; 890 mg total catechins) confection snack food while following a low-polyphenol diet for 28 days. Assessments will include measures of circulating endotoxin (primary outcome) and secondary outcomes including biomarkers of endotoxin exposure, region-specific measures of intestinal permeability, gut microbiota composition, diversity, and functions, intestinal and systemic inflammatory responses, and catechins and microbiota-derived catechin metabolites. Study outcomes will provide the first report of the GTE-mediated benefits that alleviate gut barrier dysfunction in relation to endotoxemia-associated inflammation in MetS persons. This is expected to help establish an effective dietary strategy to mitigate the growing burden of MetS that currently affects ~35% of Americans.
Keywords: BMI, body mass index; Catechin; Endotoxemia; GTE, green tea extract; Gut barrier function; Gut dysbiosis; Gut microbiota; Inflammation; LBP, LPS binding protein; LPS, lipopolysaccharides; MetS, metabolic syndrome; Metabolic syndrome; NFκB, nuclear factor κB; PCoA, principal coordinates analysis; SCFA, short chain fatty acid; TLR4, Toll-like receptor-4; TNF- α, tumor necrosis factor-α; Tea.
© 2019 The Authors.
Figures



References
-
- Aguilar M., Bhuket T., Torres S., Liu B., Wong R.J. Prevalence of the metabolic syndrome in the United States, 2003-2012. J. Am. Med. Assoc. 2015;313:1973–1974. - PubMed
-
- Alberti K.G., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A., Fruchart J.C., James W.P., Loria C.M., Smith S.C., Jr. Vol. 120. National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity, Circulation; 2009. pp. 1640–1645. (International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity, Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention). - PubMed
-
- Cani P.D., Amar J., Iglesias M.A., Poggi M., Knauf C., Bastelica D., Neyrinck A.M., Fava F., Tuohy K.M., Chabo C., Waget A., Delmee E., Cousin B., Sulpice T., Chamontin B., Ferrieres J., Tanti J.F., Gibson G.R., Casteilla L., Delzenne N.M., Alessi M.C., Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. - PubMed
-
- Neves A.L., Coelho J., Couto L., Leite-Moreira A., Roncon-Albuquerque R., Jr. Metabolic endotoxemia: a molecular link between obesity and cardiovascular risk. J. Mol. Endocrinol. 2013;51:R51–R64. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

