Comparison of the functional and structural characteristics of rare TSC2 variants with clinical and genetic findings
- PMID: 31799751
- PMCID: PMC7154745
- DOI: 10.1002/humu.23963
Comparison of the functional and structural characteristics of rare TSC2 variants with clinical and genetic findings
Abstract
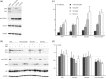
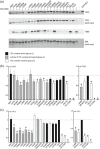
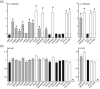
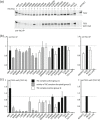
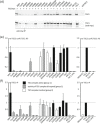
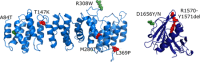
The TSC1 and TSC2 gene products interact to form the tuberous sclerosis complex (TSC), an important negative regulator of the mechanistic target of rapamycin complex 1 (TORC1). Inactivating mutations in TSC1 or TSC2 cause TSC, and the identification of a pathogenic TSC1 or TSC2 variant helps establish a diagnosis of TSC. However, it is not always clear whether TSC1 and TSC2 variants are inactivating. To determine whether TSC1 and TSC2 variants of uncertain clinical significance affect TSC complex function and cause TSC, in vitro assays of TORC1 activity can be employed. Here we combine genetic, functional, and structural approaches to try and classify a series of 15 TSC2 VUS. We investigated the effects of the variants on the formation of the TSC complex, on TORC1 activity and on TSC2 pre-mRNA splicing. In 13 cases (87%), the functional data supported the hypothesis that the identified TSC2 variant caused TSC. Our results illustrate the benefits and limitations of functional testing for TSC.
Keywords: CRISPR/Cas9; TORC1; TSC2; VUS; functional assay; tuberous sclerosis complex.
© 2019 The Authors. Human Mutation Published by Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures


References
-
- Arnold, K. , Bordoli, L. , Kopp, J. , & Schwede, T. (2006). The SWISS‐MODEL workspace: A web‐based environment for protein structure homology modelling. Bioinformatics, 22, 195–201. - PubMed
-
- Daumke, O. , Weyland, M. , Chakrabarti, P. P. , Vetter, I. R. , & Wittinghofer, A. (2004). The GTPase activating protein Rap1GAP uses a catalytic asparagine. Nature, 429, 197–201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

