Prevention of Recurrence After Recovery From a Major Depressive Episode With Antidepressant Medication Alone or in Combination With Cognitive Behavioral Therapy: Phase 2 of a 2-Phase Randomized Clinical Trial
- PMID: 31799993
- PMCID: PMC6902236
- DOI: 10.1001/jamapsychiatry.2019.3900
Prevention of Recurrence After Recovery From a Major Depressive Episode With Antidepressant Medication Alone or in Combination With Cognitive Behavioral Therapy: Phase 2 of a 2-Phase Randomized Clinical Trial
Erratum in
-
Error in Figure 2 Curves and Revisions to Subtitle, Abstract, and Figure Titles and Captions.JAMA Psychiatry. 2020 Mar 1;77(3):328. doi: 10.1001/jamapsychiatry.2019.4878. JAMA Psychiatry. 2020. PMID: 31995130 Free PMC article. No abstract available.
Abstract
Importance: Antidepressant medication (ADM) maintenance treatment is associated with the prevention of depressive recurrence in patients with major depressive disorder (MDD), but whether cognitive behavioral therapy (CBT) treatment is associated with recurrence prevention remains unclear.
Objective: To determine the effects of combining CBT with ADM on the prevention of depressive recurrence when ADMs are withdrawn or maintained after recovery in patients with MDD.
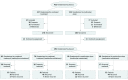
Design, setting, and participants: A total of 292 adult outpatients with chronic or recurrent MDD who participated in the second phase of a 2-phase trial. Participants had recovered in the first phase of the trial receiving ADM, either alone or in combination with CBT. The trial was conducted in research clinics in 3 university medical centers in the United States. Patients in phase 2 were randomized to receive maintenance of or withdrawal from ADM and were followed up for 3 years. The first and last patients entered phase 2 in August 2003 and October 2009, respectively. The last patient completed phase 2 in August 2012. Data were analyzed from December 2013 to December 2018.
Interventions: Maintenance of or withdrawal from treatment with ADM.
Main outcomes and measures: Recurrence of an MDD episode using longitudinal interval follow-up evaluations; sustained recovery across both phases.
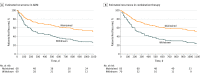
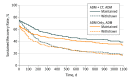
Results: A total of 292 participants (171 women, 121 men; mean [SD] age 45.1 [12.9] years) were included in analyses of depressive recurrence. Maintenance ADM yielded lower rates of recurrence compared with ADM withdrawal regardless of whether patients had achieved recovery in phase 1 with ADM alone (48.5% vs 74.8%; z = -3.16; P = .002; number needed to treat [NNT], 2.8; 95% CI, 1.8-7.0) or ADM plus CBT (48.5% vs 76.7%; z = -3.49; P < .001; NNT, 2.7; 95% CI, 1.9-5.9). Sustained recovery rates differed as a function of phase 2 condition, with maintenance ADM superior to ADM withdrawal (z = 2.90; P = .004; OR, 2.54; 95% CI, 1.37-4.84; NNT, 2.3; 95% CI, 1.5-6.4). Phase 1 condition was not associated with differential rates of sustained recovery (ADM alone vs ADM plus CBT; z = 0.22; P = .83; OR, 1.08; 95% CI, 0.52-2.11; NNT, 26.0; 95% CI, number needed to harm 3.2 to NNT 2.8), nor was there a significant interaction of phase 1 condition and phase 2 condition (z = 0.30; P = .77; OR, 1.14; 95% CI, 0.49-2.88).
Conclusions and relevance: Maintenance ADM treatment, but not previous exposure to CBT, was associated with reduced rates of depressive recurrence. In previous studies, when CBT has been provided without ADM, CBT has shown a preventive effect on depressive relapse. Whether CBT also has a preventive effect on depressive recurrence, or if adding ADM interferes with any such preventive effect, remains unclear.
Trial registration: ClinicalTrial.gov identifier: NCT00057577.
Conflict of interest statement
Figures
Comment in
-
Relapse Prevention After Recovery in Patients With Persistent Major Depressive Disorder-An Active Pursuit.JAMA Psychiatry. 2020 Mar 1;77(3):231-232. doi: 10.1001/jamapsychiatry.2019.3637. JAMA Psychiatry. 2020. PMID: 31799999 No abstract available.

