Adult loss of Cacna1a in mice recapitulates childhood absence epilepsy by distinct thalamic bursting mechanisms
- PMID: 31800012
- PMCID: PMC6935748
- DOI: 10.1093/brain/awz365
Adult loss of Cacna1a in mice recapitulates childhood absence epilepsy by distinct thalamic bursting mechanisms
Erratum in
-
Erratum.Brain. 2020 Mar 1;143(3):e24. doi: 10.1093/brain/awaa007. Brain. 2020. PMID: 32333675 Free PMC article. No abstract available.
Abstract
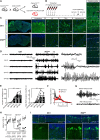
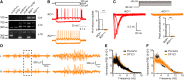
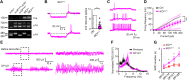
Inborn errors of CACNA1A-encoded P/Q-type calcium channels impair synaptic transmission, producing early and lifelong neurological deficits, including childhood absence epilepsy, ataxia and dystonia. Whether these impairments owe their pathologies to defective channel function during the critical period for thalamic network stabilization in immature brain remains unclear. Here we show that mice with tamoxifen-induced adult-onset ablation of P/Q channel alpha subunit (iKOp/q) display identical patterns of dysfunction, replicating the inborn loss-of-function phenotypes and, therefore demonstrate that these neurological defects do not rely upon developmental abnormality. Unexpectedly, unlike the inborn model, the adult-onset pattern of excitability changes believed to be pathogenic within the thalamic network is non-canonical. Specifically, adult ablation of P/Q channels does not promote Cacna1g-mediated burst firing or T-type calcium current (IT) in the thalamocortical relay neurons; however, burst firing in thalamocortical relay neurons remains essential as iKOp/q mice generated on a Cacna1g deleted background show substantially diminished seizure generation. Moreover, in thalamic reticular nucleus neurons, burst firing is impaired accompanied by attenuated IT. Interestingly, inborn deletion of thalamic reticular nucleus-enriched, human childhood absence epilepsy-linked gene Cacna1h in iKOp/q mice reduces thalamic reticular nucleus burst firing and promotes rather than reduces seizure, indicating an epileptogenic role for loss-of-function Cacna1h gene variants reported in human childhood absence epilepsy cases. Together, our results demonstrate that P/Q channels remain critical for maintaining normal thalamocortical oscillations and motor control in the adult brain, and suggest that the developmental plasticity of membrane currents regulating pathological rhythmicity is both degenerate and age-dependent.
Keywords: CACNA1A; CACNA1H; ataxia; burst firing; childhood absence epilepsy.
© The Author(s) (2019). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures


References
-
- Blumenfeld H. Cellular and network mechanisms of spike-wave seizures. Epilepsia 2005; 46: 21–33. - PubMed
-
- Broicher T, Kanyshkova T, Meuth P, Pape HC, Budde T. Correlation of T-channel coding gene expression, IT, and the low threshold Ca2+ spike in the thalamus of a rat model of absence epilepsy. Mol Cell Neurosci 2008; 39: 384–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

